- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Lower oral retinoid dose effective for psoriasis
A lower dose of the oral retinoid acitretin is effective for moderate-to-severe psoriasis and can minimize adverse effects, according to a study presented at the 64th Annual Meeting of the American Academy of Dermatology in San Francisco. Current practice is to administer the maximal tolerated dose of 25 mg to 50 mg acitretin daily.
A lower dose of the oral retinoid acitretin is effective for moderate-to-severe psoriasis and can minimize adverse effects, according to a study presented at the 64th Annual Meeting of the American Academy of Dermatology in San Francisco. Current practice is to administer the maximal tolerated dose of 25 mg to 50 mg acitretin daily.
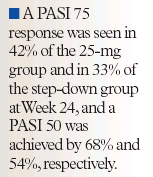
The phase 4 study randomized 77 patients to receive either 25 mg acitretin daily for 24 weeks or 25 mg acitretin daily for 12 weeks followed by a step-down regimen of 10 mg once daily for 12 weeks. The mean baseline Psoriasis Area and Severity Index (PASI) score was 21 in the 25-mg group and 19 in the step-down group. The mean percentage of body surface area involvement at baseline was 34% and 30% in the groups, respectively.
Dose adjustment was permitted with reductions to 10 mg daily among those assigned the 25-mg regimen and up to 35 mg daily for those in either group failing to achieve a 2-grade improvement in OLA at Week 12. The daily mean acitretin dose from baseline to Week 12 was 25 mg in both treatment groups, and the daily mean dose from Weeks 12 to 24 was 26 mg in the 25-mg treatment group and 25 mg in the step-down group.
At 12 weeks, 18% of patients in both treatment groups achieved an OLA of none or minimal. After 24 weeks, 45% of those in the 25-mg group and 49% of those in the step-down group achieved an OLA of none or minimal. A PASI 75 response was seen in 42% of the 25-mg group and in 33% of the step-down group at Week 24 and a PASI 50 was achieved by 68% and 54%, respectively.
There were no serious adverse events, and the most common adverse event was cheilitis in 8% of patients in both treatment groups. Two patients had isolated liver function test (LFT) abnormalities during the study: 1 after a 35-mg dose of acitretin, which resolved after the dose was decreased to 10 mg, and another in the step-down group who developed an isolated abnormality that resolved without dose adjustment. No patients in either group developed elevated triglyceride levels, and none required the initiation of lipid-lowering therapy during the study.
Scripius Removes Dupixent from Formulary
September 7th 2023The decision by Scripius (previously Select Health Prescriptions) was made based on the cost of Dupixent, as well as because of an increased demand to use Dupixent for mild atopic dermatitis when the primary patient population is those with moderate to severe disease.
