- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Management of chronic kidney disease: An emphasis on delaying disease progression and treatment options
Chronic kidney disease (CKD) remains a significant cause of morbidity and mortality. Here's a review of treatment options and guidelines to delay disease progression.

Other risk factors for developing kidney disease include but are not limited to being older than age 60 years, having a glomerular filtration rate (GFR) <60 mL/min/1.73 m2; receiving nephrotoxic medications; family history; being of African American, Hispanic, or Asian descent; smoking; and being overweight.3,4 Conditions such as glomerulonephritis, chronic interstitial nephritis, chronic pyelonephritis, polycystic kidney disease, and intratubular obstruction can also lead to CKD.3 Left untreated, CKD can lead to significant morbidity and mortality from one of many complications, such as cardiovascular disease, anemia, electrolyte imbalances, bone disease, and kidney failure. Mortality in patients with end-stage renal disease (ESRD) remains 10 to 20 times higher than in the general population. Thus, the focus continues to be on optimizing the care of patients with CKD before they reach the onset of ESRD.5
National Health and Nutrition Examination Survey (NHANES) data show that 23 million adults (11.5%) suffered from CKD in 2004. Although CKD was more common in women, men with CKD were 50% most likely to fully progress to kidney failure.6 Data from the US Renal Data System (USRDS) show the prevalence of kidney failure has more than doubled in the United States over the past decade. Adjusted all-cause hospitalization and mortality rates are higher in CKD patients than in those without the disease, with hospitalization rates being 40% higher and mortality rates 39% higher.7 In 2007, more than 500,000 Americans underwent treatment for ESRD, at a cost of $35.32 billion. Additionally, 111,000 patients initiated treatment for ESRD. The National Institutes of Health has estimated that 2 million ESRD patients will receive treatment by 2030.8
CKD often goes undiagnosed and therefore remains undertreated. The condition can be identified by a blood test for creatinine, which is used to calculate a patient's GFR, or level of kidney function. Higher levels of creatinine may indicate a lower GFR and thus a decreased capability of the kidneys to excrete waste. Creatinine levels, however, may present as normal or only slightly abnormal in early stages of CKD. This is because before creatinine shows as elevated outside the normal range, at least half the renal function must be lost.5 In short, serum creatinine is affected by the level of GFR and other factors independent of GFR, including age, gender, race, body size, diet, nephrotoxic medications, and laboratory analytical methods.4 For this reason the use of serum creatinine alone may not be sufficient for determining CKD. Proteinuria is an early and sensitive marker of kidney damage in many types of CKD. It is important to assess and monitor proteinuria because increased levels are associated with a faster progression of kidney disease, and it also is a key indicator of increased patient risk for cardiovascular disease. Proteinuria can also be used to demonstrate effectiveness of therapy, and can help identify the origin of kidney disease.4,9
The type of protein in the urine can assist in establishing the underlying cause of CKD. Increased amounts of globulin can indicate renal tubulointerstitial disease, whereas increased albumin excretion can be due to glomerular disease. Albumin is a sensitive marker of kidney damage and can be indicative of the most common causes of CKD, which are diabetes and hypertension. The measure of albumin normally present in the urine is less than 30 mg. Microalbuminuria refers to the presence of 30 mg to 300 mg of albumin in the urine. Macroalbuminuria is albumin in the urine at greater than 300 mg.10,11 To fully investigate the underlying cause of kidney damage, various forms of medical imaging, blood tests, or renal biopsy are used to determine if the cause may be reversible.
DISEASE CLASSIFICATION
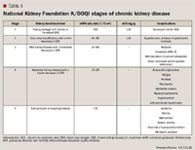
Stage 1 of CKD is the mildest, usually causing few symptoms, whereas stage 5, kidney failure, is the most severe, leaving patients with a poor life expectancy if left untreated. Stage 5 is also referred to as "established" CKD (also called ESRD, chronic kidney failure, and chronic renal failure).4,9
ESTIMATING GFR


Use of the newer CKD-EPI equation results in a lower prevalence of eGFR <60 mL/min/1.73 m2 for most groups compared with the eGFR obtained with the MDRD equation. Regardless of which method is used to estimate GFR, low eGFR and high urinary ACR are most often found in the group older than 60 years of age and in those groups with self-reported diabetes, hypertension, and cardiovascular disease.13 These calculation methods are not optimal when used for patients with acute renal failure because a stable creatinine level is required to ensure accuracy. GFR typically declines with age, making it difficult to differentiate age-related decreases in GFR from actual kidney disease. For that reason, more detailed clinical evaluations of elderly patients, including blood pressure and ACR, should be considered.
DELAYING CKD PROGRESSION
Detecting CKD early is important to help delay its progression, reduce associated complications, and decrease adverse effects of the disease. Tight glycemic control, blood pressure control, and reducing albuminuria can help slow the progression of CKD.10,11,16 Other data suggest that reducing dietary protein, implementing therapy for hyperlipidemia, and treating anemia may also have an impact on the progression of CKD.4,5
Controlling diabetes. Diabetes is one of the main contributors to development of CKD, and tight glycemic control can help reduce renal complications. The Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) showed that careful control of blood glucose reduced the development of microalbuminuria and nephropathy in patients with type 1 and type 2 diabetes.17,18 Adequate control of blood pressure with antihypertensive agents such as angiotensin-converting enzyme inhibitors (ACEIs) has been shown to delay the progression of albuminuria in both type 1 and type 2 diabetes. These agents also demonstrate renoprotective effects in nephropathy due to type 2 diabetes.19,20
Patients with diabetes should be instructed to monitor their blood glucose at least 2 to 4 times a day; however, the frequency and timing should be dictated specifically by the particular needs and goals of the patient.21 Patients should also receive additional education regarding their diet, the importance of exercise and weight control, and should be monitored closely for medication adherence. A1C(also known as HbA1c, or glycosylated hemoglobin) should be obtained twice annually for patients at treatment goal, and quarterly for those not at treatment goal or when their medication therapy has changed.21 Guidelines recommend an A1Cgoal of <7.0% for most diabetic patients; however certain populations (eg, children, elderly) may require special consideration as they may be more prone to hypoglycemia. Screening for albuminuria is recommended at a minimum of once annually to assess therapy response and to monitor for nephropathy.21 Patients presenting with microalbuminuria (2 or more ACRs above 30 mg/g at 2 separate readings) should be treated to reduce the risk of CKD and cardiovascular disease.11
Controlling hypertension. Hypertension is a well-established cause, a common complication, and an important risk factor for progression of CKD. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7), the American Diabetes Association (ADA), and the NKF all recommend a blood pressure level of less than 140/90 mm Hg in the general population and less than 130/80 mm Hg in patients with CKD or diabetes.4,9,21,22 The NKF Task Force on Cardiovascular Disease in Chronic Renal Disease recommends an even greater reduction in blood pressure if the urine protein:creatinine ratio is 500 mg or more. This is based on data showing that the degree of blood pressure control is correlated with the decreased development of microalbuminuria.9
In the general population, diuretics and beta-blockers are recommended because of their efficacy in reducing cardiovascular mortality and morbidity.22 However, the risk for incident diabetes is shown as higher in those taking a thiazide diuretic compared with those who do not and also in those taking beta-blockers compared with those using another antihypertensive category.23 ACEIs and calcium-channel antagonists are also effective in the general population. ADA guidelines state that pharmacologic therapy for patients having both diabetes and hypertension should include either an ACEI or an angiotensin-receptor blocker (ARB). If needed to achieve further blood pressure control, a thiazide diuretic should be added for those patients with an estimated GFR of =30 mL/min/1.73 m2 and a loop diuretic for those with an estimated GFR <30 mL/min/1.73 m2.2,24
As stated above, multiple antihypertensive agents may be used for CKD patients, but those most effective in decreasing the progression of CKD are the ACEIs, ARBs, and nondihydropyridine calcium-channel blockers (eg, verapamil and diltiazem) due to their ability to delay the advance of CKD independent of their blood pressure-lowering effect.9
Controlling proteinuria. Proteinuria is a key predictor of CKD progression, and slowing the decline in kidney function for both diabetic and nondiabetic patients can be accomplished by reducing urinary protein excretion. ACEIs and ARBs are effective antihypertensive agents that also benefit CKD patients by reducing proteinuria, the decline in GFR, and progression to ESRD.1
Mineral and electrolyte disorders in CKD. As kidney disease progresses, gradually the kidneys become incapable of maintaining normal fluid and electrolyte balance, leaving the body unable to excrete excess water, electrolytes, and metabolic waste products or to produce sufficient amounts of the renal hormones calcitriol and erythropoietin. As the number of functioning nephrons continues to decline, the kidneys become unable to maintain sodium balance. If a patient's sodium intake exceeds the excretory ability of the kidneys, edema and hypertension results. Therefore, it is most often necessary to restrict sodium intake for patients with progressing CKD. As kidney failure progresses, the kidney becomes unable to dilute the urine and in turn excrete excess water, resulting in lower than normal serum sodium concentrations (hyponatremia) despite sodium retention.
The normal kidney excretes the majority of potassium taken in by diet. Patients with progressing CKD and subsequent renal failure retain potassium and develop hyperkalemia (serum potassium >5 mEq/L).9 NKF K/DOQI guidelines report incidence rates as low as <1% to as high as 62.5% (incidence higher with ACEI use than with ARBs). Although mild hyperkalemia can be tolerated by patients with CKD, further increases in serum potassium can be life threatening, putting patients at risk for cardiac dysrhythmias and a subsequent need for dialysis. Measures to lower serum potassium can be used to prevent or treat hyperkalemia. These include discontinuation or dose-reduction of medications that raise serum potassium, prescription of a low-potassium diet (restricting dietary potassium to 40 to 60 mEq/day), loop diuretics, alkali replacement (if metabolic acidosis, serum bicarbonate concentration <21 mEq/L), or treatment with sodium polystyrene sulfonate (SPS).4,9 SPS is a cation exchange resin that trades sodium ions for potassium ions, acting mostly in the large intestine, to enhance the fecal elimination of excess potassium. SPS typically is used in combination with a cathartic agent (eg, sorbitol) to enhance fecal elimination of potassium as well as to prevent constipation that can often be caused by the SPS therapy. However, concomitant use of SPS and sorbitol has been linked to an increased risk for gastrointestinal injuries, and product labeling includes this warning.25 In asymptomatic CKD patients with mild hyperkalemia, chronic administration of SPS is useful in preventing further elevations of serum potassium, but use for acute management should be avoided as it causes slow elimination of total body potassium over hours to days, resulting in a slower onset of action.26 The NKF K/DOQI guidelines recommend SPS use in CKD and ESRD for the management of mild to moderate elevations in serum potassium concentrations in asymptomatic patients. As patients progress to higher stages of CKD, dialysis should be considered.27
At stage 5 (ESRD), CKD can begin to have an effect on bone metabolism, often leading to a complex bone disease known as renal osteodystrophy. The alterations in bone metabolism in CKD are due primarily to a reduction in calcitriol production, hypocalcemia, and increased phosphate and parathyroid hormone (PTH; secondary hyperparathyroidism). The increased excretion of phosphate by the kidney results in low activated vitamin D3levels (due to the inability of the kidney to convert vitamin D3into its active form, calcitriol), and can result in further hypocalcemia. The increased PTH stimulates the activity of osteoclasts, the cells that break down bone. Osteoclastic bone resorption causes more calcium to be released from the bone into the bloodstream. In addition to elevated blood calcium levels, there is a resulting loss of bone mass, a weakening of bones, and the formation of cyst-like tumors in and around the bone.3,28 Treatment for renal osteodystrophy includes calcium and vitamin D supplementation, dietary phosphate restriction, use of phosphate binders such as calcium carbonate, calcium acetate, sevelamer hydrochloride, or lanthanum carbonate, or cinacalcet. Poor response may lead to renal transplantation.
TREATMENT OPTIONS FOR BONE METABOLISM AND DISEASE IN CKD
Hypocalcemia. Deficiencies of calcitriol and calcium can be corrected mostly through oral administration. Calcitriol is available as oral capsules (0.25 and 0.50 µg), an oral solution (1 µg/mL), and as an intravenous solution (1 µg/mL). The optimal daily dose must be carefully determined for each patient. Calcitriol can be dosed once daily or every other day, but should always be started at the lowest possible dose and not increased without careful monitoring of serum calcium. Serum levels of corrected total calcium should be maintained within the normal range, preferably towards the lower end (8.4 to 9.5 mg/dL [2.10 to 2.37 mmol/L]).4,29,30 The effectiveness of calcitriol therapy depends on the patient also receiving an adequate but not excessive daily intake of calcium.30 The NKF K/DOQI guidelines on bone metabolism and disease in CKD suggest total elemental calcium intake not exceed 2,000 mg/d and that the calcium intake should comprise no more than 1,500 mg from the binder and no more than 500 mg from diet.3
A variety of oral calcium supplements are available. These include calcium carbonate and calcium acetate, which although are used mostly as phosphate binders, do provide significant amounts of calcium. Calcium citrate is another product that has a much higher bioavailability than the calcium carbonate and acetate formulations. The citrate formulation provides an additional benefit as its metabolized form (bicarbonate) results in the generation of a buffer for patients.31
Hyperphosphatemia. Patients with CKD are unable to effectively excrete phosphate. Even with dietary restriction, phosphate can continue to accumulate in the bodily fluids of patients with renal failure leading to hyperphosphatemia (serum phosphorus level >5.5 mg/dL). As described earlier, high serum levels of phosphate can lead to hyperparathyroidism, renal bone disease, vascular calcification, and increased mortality.32
Management of hyperphosphatemia includes reduction of dietary intake of phosphate, inhibition of intestinal phosphate absorption with phosphate binders, and removal of phosphate through the use of dialysis. Restriction of dietary phosphorus is difficult as high concentrations of phosphorus are commonly found in foods including dairy products, nuts, and meat that are also necessary to assure adequate dietary protein intake to prevent malnutrition. Therefore, most patients with CKD will require phosphate-binding medications, which is the mainstay of therapy for managing hyperphosphatemia.32

Although use of calcium-based phosphate binders is cost effective, long-term safety concerns exist regarding their potential role in the progression of cardiovascular calcification. Sevelamer hydrochloride has been recommended as an alternative noncalcium phosphate binder. Results from the Calcium Acetate Renagel Evaluation (CARE) study indicate that patients with CKD on hemodialysis are more effectively treated with calcium acetate compared with sevelamer and more frequently achieved the K/DOQI treatment goals for serum phosphorus, whereas the Treat-to-Goal study found sevelamer slowed the progression of coronary calcification in patients on hemodialysis compared to calcium-based phosphate binders.32,41 Cost-benefit analysis currently favors calcium acetate as a first-line therapy choice, with further study needed regarding the benefits of sevelamer on cardiovascular mortality.
Secondary hyperparathyroidism. Secondary hyperparathyroidism is a frequent complication of CKD caused by the drop in circulating calcitriol and subsequent disturbances in calcium and phosphate metabolism. As described above, elevated PTH stimulates the activity of osteoclasts, which are the cells that break down bone. Osteoclastic bone resorption causes calcium release from the bone into the bloodstream. Therefore, limiting the elevation of PTH is an important treatment focus for CKD patients. In addition to the traditional therapies mentioned above (phosphate binders, vitamin D, and calcium supplementation), vitamin D analogs (calcitriol analogs) and calcimimetics can be used for the treatment of secondary hyperparathyroidism.
Vitamin D analogs are structurally similar to calcitriol. They have the ability to suppress PTH synthesis and release and have a low incidence of raising serum calcium or phosphorus. Because they do have some of the same actions as vitamin D, they are contraindicated in patients at risk for hypercalcemia or in those with vitamin D toxicity. Paricalcitol and doxercalciferol are 2 vitamin D analogs available in both oral and intravenous formulations for the prevention and treatment of secondary hyperparathyroidism associated with CKD.
Calcimimetics work to increase the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. The calcium-sensing receptor on the surface of the chief cell of the parathyroid gland is the principal regulator of PTH synthesis and secretion. Calcimimetics work to directly lower PTH levels by increasing the sensitivity of the calcium-sensing receptor to extracellular calcium. The reduction in PTH is associated with a related decrease in serum calcium levels. Treatment with this agent should not be started in patients with low calcium levels. During use, repeat blood tests to monitor calcium, phosphorus, and intact PTH levels should be performed. Cinacalcet was the first calcimimetic agent approved by the FDA for treatment of secondary hyperparathyroidism in dialysis patients.42
Anemia in CKD. The kidney is responsible for the synthesis of erythropoietin, a peptide hormone that stimulates the proliferation of red blood cells in the bone marrow. As kidney disease progresses, erythropoietin production begins to decline. Anemia of chronic renal disease begins when the GFR falls below 30% to 35% of normal. It is unclear if anemia accelerates the progression of renal disease, but it is independently associated with the development of left ventricular hypertrophy and other cardiovascular complications. Treatment of anemia with recombinant human erythropoietin may possibly slow progression of chronic renal disease. Current NKF guidelines recommend evaluation of anemia when hemoglobin is <11 g/dL and consideration of recombinant human erythropoietin if hemoglobin is consistently <11 g/dL, to maintain a target hemoglobin level of 11 to 12 g/dL.43 According to guidelines, CKD patients having a hematocrit <33% (normal being 45% and 40% for men and women, respectively) or hemoglobin <11 g/dL should be treated with an erythropoiesis-stimulating agent (ESA) such as epoetin alpha or darbepoetin alfa.4
However, FDA recently issued a drug safety communication regarding the use of ESAs in CKD that included modified dosing recommendations. This communication called for more conservative dosing of ESAs as data showed increased risks of cardiovascular events with ESAs in this patient population. The new dosing recommendations were based on clinical trials showing that using ESAs to target a hemoglobin level of greater than 11 g/dL in patients with CKD provides no additional benefit than lower target levels and increases the risk for serious adverse cardiovascular events, such as heart attack or stroke.44 Manufacturers of ESAs have revised the warnings and precautions and dosage and administration sections of the package labeling to include this new information. Healthcare professionals should weigh the possible benefits of using ESAs in CKD patients against the increased risk for serious cardiovascular events.
AGENTS IN THE PIPELINE FOR CKD AND RELATED CONDITIONS

Nrf2 pathway inducers. Bardoxolone methyl (also known as "RTA 402" and "CDDO-methyl ester") was the first antioxidant inflammation modulator under study and has been advanced to late-stage clinical trials for the treatment of patients with CKD and type 2 diabetes. Growing evidence suggests that in CKD, oxidative stress and excessive inflammation have multiple deleterious effects on glomerular structure and function, which drive disease progression. Short-term effects of oxidative stress and inflammation include mesangial cell contraction and glomerular endothelial dysfunction. As these effects progress, hypertrophy of the mesangium occurs and the glomerular basement membrane thickens. This reduces the glomerular surface area available for filtration, increases intraglomerular pressure, and results in a decline in GFR and kidney function.45,53
Bardoxolone methyl is a potent known inducer of the Nrf2 pathway and works to suppress both oxidative stress and inflammation. Bardoxolone methyl has been shown to significantly increase eGFR and to improve several other markers of kidney function (eg, blood urea nitrogen, uric acid) in patients with CKD, which correlate with the improvement in eGFR.46 A phase 2 clinical study presented at the 2011 World Congress of Nephrology demonstrated that the drug produced a significant increase in eGFR.45,47 Clinical observations in phase 2 studies have also demonstrated parallel improvements in other kidney function measures, including blood urea nitrogen and uric acid, which correlate with the improvement in eGFR.45
This multicenter, open-label, dose-ranging study enrolled 130 patients with moderate to severe CKD (eGFR, 15–45 mL/min/1.73 m2) and type 2 diabetes to receive 2.5, 5, 10, 15, or 30 mg of bardoxolone methyl administered orally, once daily for 12 weeks. The primary and secondary efficacy objectives of establishing dose-related trends in eGFR change from baseline to weeks 4 and 12 were met. Patients receiving 5 mg demonstrated modest activity at both time points, while patients receiving 15 and 30 mg experienced eGFR increases from baseline that were clinically and statistically significant (P<.005) at week 4 (7.7 and 8.2 mL/min/1.73 m2, respectively) and week 12 (7.3 and 11.1 mL/min/1.73 m2, respectively). In the phase 2 study, bardoxolone methyl was generally well tolerated and found suitable for once-daily dosing. Adverse effects most commonly reported (8.6%) included muscle spasms, fatigue, peripheral edema, hypoglycemia, nausea, and diarrhea.47
The 15-mg dose from the phase 2 study was found to provide an adequate balance between efficacy and safety and was chosen for use in a planned phase 3 follow-up study. This multinational, double-blind, placebo-controlled phase 3 outcomes study (BEACON) is currently ongoing, testing bardoxolone methyl's impact on progression to ESRD or cardiovascular death in 1,600 patients with stage 4 CKD (eGFR, 15–30 mL/min/1.73 m2) and type 2 diabetes, with results expected in 2013.48
Glycosaminoglycans. Sulodexide is a highly purified mixture of glycosaminoglycans (GAGs) composed of low-molecular-weight heparin (80%) and dermatan sulfate (20%). Sulodexide is used for the prophylaxis and treatment of thromboembolic diseases; however, recent research has demonstrated beneficial effects in the treatment of diabetic nephropathy.49 Nephropathy is a multifactorial complication of diabetes that can lead to chronic renal insufficiency and an increased risk of cardiovascular death. Besides oxidative stress and hemodynamic changes, GAGs are also implicated in the onset of glomerular abnormalities. GAG replacement therapy was investigated early on for the treatment of diabetic nephropathy. Sulodexide is the most researched GAG agent for reduction of albuminuria in diabetic patients.
Thirty type 1 and type 2 diabetes patients were treated with 50 mg daily oral sulodexide for 12 months; 30 matched diabetic patients served as the control group. The effects of oral sulodexide for the treatment of patients with diabetic nephropathy were evaluated. At 12 months, albuminuria was greatly reduced in the patients treated with sulodexide and increased in the control group (260% and +29% vs. baseline, respectively; P=0.0001).49 Sulodexide showed activity in both type 1 and type 2 diabetes and in both micro- and macroalbuminuric patients. There was no change in metabolic control and no systemic side effects reported in the study patients. Sulodexide was shown to greatly reduce albuminuria and to have the potential to delay progression from incipient to overt nephropathy.
Erythropoiesis-stimulating agents. FDA recently approved peginesatide, an erythropoiesis-stimulating agent (ESA), co-developed by Affymax and Takeda. Peginesatide is the first once-monthly ESA available for the treatment of anemia, a condition in which the body does not have enough healthy red blood cells, associated with CKD patients on dialysis. Peginesatide works by stimulating the bone marrow to produce more red blood cells, usually measured as hemoglobin levels, to reduce the need for transfusions in patients with CKD.50
Peginesatide is a synthetic, PEGylated peptidic compound that binds to and activates the erythropoietin receptor, thus acting as an ESA. The NDA included data from 2 randomized, active-controlled, open-label, multicenter clinical trials (EMERALD 1 and 2) that evaluated the efficacy and safety of peginesatide in maintaining hemoglobin levels. In the studies, 1,608 CKD patients on dialysis who were receiving stable doses of epoetin were randomized to receive once-monthly peginesatide or continue treatment with epoetin (dosed more frequently). EMERALD findings suggested that once-monthly peginesatide was comparable to epoetin in maintaining hemoglobin levels in CKD patients on dialysis with anemia and had a similar adverse event rate. The most common adverse events reported in 10% or more of the dialysis patients were diarrhea, vomiting, high blood pressure (hypertension), and joint, back, leg, or arm pain (arthralgia). The frequency of serious adverse events was reported to be similar between the 2 EMERALD treatment groups.51 The peginesatide phase 3 trials were the largest to support the registration of an ESA for the treatment of anemia in CKD and the first to prospectively evaluate the cardiovascular safety of an ESA using an analysis of independently evaluated cardiovascular events.
Labeling for peginesatide includes a boxed warning for CKD patients, similar to that required for other ESAs. In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered ESAs to target a hemoglobin level of greater than 11 g/dL. No trial identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. It is recommended to use the lowest peginesatide dose sufficient to reduce the need for red blood cell transfusions. Peginesatide labeling also includes a Medication Safety Program, also known as Risk Evaluation Mitigation Strategy (REMS). REMS is a strategy and plan to manage and assess a known or potential serious risk (in this instance cardiovascular risk) associated with a drug or biological product and is required if FDA finds it necessary to ensure that the benefits of a product outweigh the risks. The peginesatide FDA-approved REMS program includes a Communication Plan (provider letter and web-based educational materials), a Medication Guide, and REMS assessments that the company must submit to FDA.52,54
CONCLUSION
CKD is a global health problem and a significant cause of morbidity and mortality. Diabetes and hypertension are the largest contributors to CKD risk and cause the majority of cases. Complications and comorbidities can begin to occur very early in the stages of the disease. Early in the course of CKD, mineral and electrolyte disturbances can be treated to slow the quick progression of complications. Strategies for effective management of CKD include screening patients at high risk, controlling the progression of disease, identifying and correcting reversible factors, and preventing complications. As kidney disease progresses, it is imperative to assess patients for complications including anemia, bone metabolism abnormalities, metabolic acidosis, and malnutrition. Implementation of these important strategies in the care of CKD patients can change the course of the disease and improve long-term patient outcomes.
Ms Crockell is director of clinical pharmacy, American Health Care, Rocklin, Calif.
Disclosure Information: The author reports no financial disclosures as related to products discussed in this article.
REFERENCES
1. Yu HT. Progression of chronic renal failure. Arch Intern Med. 2003;163:1417–1429.
2. National Kidney Foundation. About chronic kidney disease. http://www.kidney.org/kidneydisease/aboutckd.cfm. Accessed April 4, 2012.
3. National Kidney Foundation. K/DOQI Clinical Handbook. CKD Stages 3-4: Bone Metabolism and Disease in Chronic Kidney Disease. 2005;1:18.
4. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S246.
5. Parmar MS. Chronic renal disease. Early identification and active management of patients with renal impairment in primary care can improve outcomes. BMJ. 2002;325:85–90.
6. National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/nchs/nhanes.htm. Updated Jan. 30, 2012. Accessed February 3, 2012.
7. United States Renal Data System. 2011 USRDS Annual Data Report: Introduction, Volume 1. Atlas of Chronic Kidney Disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: 2011;1–36. Available at: http://www.usrds.org/2011/pdf/v1_00_intro_11.pdf. Accessed February 11, 2012.
8. Schoolwerth AC, Engelgau MM, Hostetter TH, Rufo KH, Chianchiano D, McClellan WM, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. [serial online] 2006 Apr [date cited]. Available at: http://www.cdc.gov/pcd/issues/2006/apr/05_0105.htm. Accessed February 3, 2012.
9. National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–S290.
10. Levey AS. Nondiabetic kidney disease. N Eng J Med. 2002;347:1505–1511.
11. Remuzzi G, Schieppati A, Ruggenenti P.Nephropathy in patients with type 2 diabetes.N Eng J Med. 2002;346:1145–1151.
12. Naughton CA. Chronic kidney disease: early detection and treatment to delay progression. US Pharm. 2004:11:HS-27–HS-33. Available at: http://legacy.uspharmacist.com/index.asp?show=article&page=8_1386.htm Accessed February 10, 2012.
13. United States Renal Data System. 2011 USRDS Annual Data Report: Chapter 1. Chronic Kidney Disease in the General Population. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; 2011:37–44. Available at: http://www.usrds.org/2011/pdf/v1_ch01_11.pdf. Accessed February 14, 2012.
14. Matsushita K, Mahmoodi BK, Woodward M, et al for the Chronic Kidney Disease Prognosis Consortium. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012; 307(18): 1941-1951.
15. Levey AS, Stevens LA, Schmid CH, et al, for the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
16. American Diabetes Association. Position statement: nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83.
17. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
18. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
19. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462.
20. Manley HJ. Role of angiotensin-converting-enzyme inhibition in patients with renal disease. Am J Health-Syst Pharm. 2000;57(Suppl 1):S12–S18.
21. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2004;27(Suppl 1):S15–S35.
22. Chobanian AV, Bakris GL, Black HR, et al, and the National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572.
23. Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29:1065–1070.
24. American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(Suppl 1):S11–S61.
25. Kayexalate (sodium polystyrene sulfonate) [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC, December 2010.
26. Gruy-Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol. 1998;9:1924–1930.
27. National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Available at: http://www.kidney.org/professionals/kdoqi/guideline_uphd_pd_va/hd_guide1.htm Accessed April 4, 2012.
28. National Kidney Foundation. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int. 2009;76(Suppl 113):S3–S8.
29. National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_bone/guidestate.htm. Accessed April 4, 2012.
30. Rocaltrol (calcitriol) [package insert]. Parsippany, NJ: Validus Pharmaceuticals, August 2010.
31. Facts & Comparisons, Wolters Kluwer Health, 2012. Available at: http://online.factsandcomparisons.com/MonoDisp.aspx?monoID=fandc-hcp13096&quick=178938%7c5&search=178938%7c5&isstemmed=True#firstMatch/. Accessed April 10, 2012.
32. Nolan CR, Qunibi WY. Treatment of hyperphosphatemia in patients with chronic kidney disease on maintenance hemodialysis. Kidney Int Suppl. 2005;95:S13–S20.
33. Rose BD. Electrolyte complications of antacid therapy. UpToDate, Wolters Kluwer Health, 2012. Available at: http://www.uptodate.com/contents/electrolyte-complications-of-antacid-therapy. Accessed April 10, 2012.
34. Facts & Comparisons, Wolters Kluwer Health, 2012. Available at: http://online.factsandcomparisons.com/MonoDisp.aspx?monoID=fandc-hcp13144&quick=240961%7c5&search=240961%7c5&isstemmed=True#firstMatch/. Accessed April 10, 2012.
35. PhosLo Gelcaps (calcium acetate) [package insert]. Waltham, MA: Fresenius Medical Care North America, January 2007.
36. Phoslyra (calcium acetate) [package insert]. Waltham, MA: Fresenius Medical Care North America, April 2011.
37. Renvela (sevelamer carbonate) [package insert]. Cambridge, MA: Genzyme Corporation, May 2011.
38. Renagel (sevelamer hydrochloride) [package insert]. Cambridge, MA: Genzyme Corporation, November 2007.
39. Fosrenol (lanthanum carbonate) [package inert]. Wayne, PA: Shire US Inc., August 2011.
40. National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_bone/guide4.htm. Accessed April 4, 2012.
41. Huybrechts KF, Caro JJ, London GM. Modeling the implications of changes in vascular calcification in patients on hemodialysis. Kidney Int. 2005;67:1532–1538.
42. Sensipar (cinacalcet) [package insert]. Thousand Oaks, CA: Amgen Inc., August 2011.
43. National Kidney Foundation. NKF–KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:474.
44. FDA drug safety communication. Modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease, June 24, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Accessed January 31, 2012.
45. Reata Pharmaceuticals pipeline overview. Bardoxolone methyl. Available at: http://www.reatapharma.com/pipeline/bardoxolone-methyl.aspx. Accessed February 15, 2012.
46. Reata Pharmaceuticals press release. Reata to present at Jefferies 2010 Global Life Sciences Conference. June 9, 2010. Available at: http://www.reatapharma.com/news_detail.asp?id=60/. Accessed February 15, 2012.
47. Pergola PE, Grossman EB, Krauth M, Ruiz S, Meyer CJ. Results from a phase 2 study of an amorphous spray-dried dispersion (sdd) formulation of bardoxolone methyl. Presented at: World Congress of Nephrology, Vancouver, Canada, April 11, 2011. Available at: http://www.abstracts2view.com/wcn/view.php?nu=WCN11L_1788&terms/. Accessed February 15, 2012.
48. ClinicalTrials.gov/. NCT01351675. Bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes (BEACON). Available at: http://www.clinicaltrials.gov/ct2/show/NCF01351675?term=bardoxolone+BEACON&rank=1/. Accessed February 15, 2012.
49. Achour A, Kacem M, Dibej K, Skhiri H, Bouraoui S, El May M. One year course of oral sulodexide in the management of diabetic nephropathy. J Nephrol. 2005;18:568–574.
50. FDA news release: FDA approves Omontys to treat anemia in adult patients on dialysis, March 27, 2012. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm297464.htm. Accessed April 10, 2012.
51. Affymax, Inc. press release. Affymax and Takeda announce FDA acceptance of new drug application for peginesatide. July 27, 2011. Available at: http://www.investors.affymax.com/releasedetail.cfm?ReleaseID=594320/. Accessed February 15, 2012.
52. Omontys (peginesatide) [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc., March 2012.
53. Pergola PE, Raskin P, Toto RD, et al, for the BEAM study investigators. Bardoxolone Methyl and kidney function in CKD with Type 2 Diabetes. NEJM. 2011; 365:327-336. Available at: http://www.nejm.org/doi/full/10.1056/NEJMoa1105351#t=articleBackground/. Accessed: May 25, 2012.
54. Affymax NDA 202799. OMONTYS (peginesatide) Injection An erythropoiesis-stimulating agent (ESA). Initial Food and Drug Administration REMS approval March 2012. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM297628.pdf. Accessed May 25, 2012.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
