- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Management of epoetin alpha use in the intensive care unit: a drug use evaluation
This study evaluates the appropriateness and cost implications of using epoetin alpha for transfusion reduction in Hartford Hospital's (Hartford, Conn) intensive care units (ICUs), with the goal of implementing a protocol for use in this setting. We conducted a literature review to determine the efficacy, safety, and clinical outcomes of epoetin alpha for transfusion reduction in the ICU. We also evaluated the safety and supply of red blood cell (RBC) transfusions and the cost considerations of epoetin alpha. The literature review demonstrated that epoetin alpha can reduce blood transfusions in the ICU setting but its use provided no difference in mortality or any other clinical outcome. Our epoetin alpha expenditure for transfusion reduction was $112,067 annually to theoretically save $14,349 in blood transfusion costs. The pharmacy and therapeutics (P&T) committee subsequently recommended that epoetin alpha not be used for transfusion reduction in the ICUs and requested that a drug use evaluation (DUE) be performed to monitor compliance, adverse effects, and cost avoidance. One year after implementation of the epoetin alpha DUE program, the compliance rate was >90%, there were no reported adverse events with blood transfusions or problems with blood supply, and a cost avoidance of $104,562 was realized. (Formulary. 2006;41:442?449.)
Abstract
Epoetin alpha (Procrit, Ortho Biotech; and Epogen, Amgen) is a recombinant human erythropoietin product that stimulates the endogenous production of red blood cells. FDA initially approved epoetin alpha for the treatment of anemia associated with chronic renal failure in 1993. Its use for this chronic indication has had a major impact in reducing blood transfusions and improving the quality of life for this patient population.1–3 Epoetin alpha subsequently received FDA approval for the treatment of anemia associated with chemotherapy, anemia associated with the use of zidovudine for human immunodeficiency virus (HIV) infection, and for the treatment of anemia in elective, noncardiac, and nonvascular surgery patients to reduce the need for blood transfusions.4,5
In November 2003, the pharmacy and therapeutics (P&T) committee recommended that the Drug Information Center (DIC) of the Department of Pharmacy Services review the use of epoetin alpha for transfusion reduction in the ICUs because of the non-FDA-approved status for the indication, lack of outcomes data for this indication at our institution, and the high cost.
The DIC, in collaboration with both the directors of transfusion services and surgical critical care, evaluated the appropriateness and cost implications of using epoetin alpha for transfusion reduction in the ICUs with the goal of developing and implementing a protocol for use in this setting.
LITERATURE REVIEW OF EPOETIN ALPHA FOR TRANSFUSION REDUCTION
At the time of the drug use evaluation (DUE), 5 randomized, controlled studies on the use of epoetin alpha in adults in ICUs had been published.6–10 However, only 2 studies, conducted by Corwin et al,6,7 were designed to assess the impact of epoetin alpha on transfusion reduction. Of the remaining 3 studies, 2 demonstrated stimulation of erythropoiesis and/or an increase in serum erythropoietin levels when epoetin alpha was administered to patients in the ICUs.8,9 The last study, a randomized, controlled trial, did not demonstrate a statistically significant difference in hemoglobin or hematocrit between the epoetin alpha treatment group and the placebo group.10
The first study performed by Corwin et al6 that assessed the impact of epoetin alpha on transfusion reduction was a prospective, multicenter, double-blind, placebo-controlled, randomized trial. Patients were randomized to receive epoetin alpha 300 units/kg or placebo subcutaneously daily for 5 days, followed by a dosing schedule of every other day for a minimum of 2 weeks or until discharge and a maximum of 6 weeks. All patients received oral iron (150 mg elemental) or parenteral iron; the dose of parenteral iron was not described. The 2 primary end points were: the cumulative blood transfusion requirements from study Day 1; and the transfusion independence between study Day 8 and study Day 42.
A total of 160 patients were enrolled in the study, with 80 patients in each group. All patients were followed up for a total of 42 days from the day of randomization, unless death occurred earlier. The epoetin alpha group had a statistically significant decrease in the total number of red blood cells (RBCs) transfused compared with the placebo group (166 versus 305, respectively; P<.002). A total of 45% of patients in the epoetin alpha group either received a blood transfusion between study Days 8 and 42 or died before study Day 42, compared with 55% of patients in the placebo group (RR=0.8; 95% CI, 0.6–1.1). There was no statistically significant difference observed between the groups in terms of mortality or incidence of adverse effects.
Some limitations of this study included: small number of patients, transfusion criteria not provided, hemoglobin value or other criteria for the decision to transfuse patients not documented, and length of ICU stay not provided (transfusion rates can vary based on length of ICU stay). In addition, because the study used many exclusion criteria, only 18% of critically ill patients met the inclusion criteria, with only half of these patients being enrolled in the study.
In another prospective, randomized, double-blind, placebo-controlled, multicenter trial, Corwin et al7 again demonstrated a reduction in the number of transfusions in critically ill patients using epoetin alpha. This study evaluated 40,000 units of epoetin alpha administered weekly beginning on the third ICU day for up to 4 doses. Patients received at least 150 mg of elemental iron either orally or via nasogastric tube beginning on study Day 1. Parenteral iron was administered to patients demonstrating an inadequate response to oral iron. As in the previous study, the authors did not describe the parenteral iron dose. The primary efficacy outcome was transfusion independence between study Days 1 and 28. Secondary end points were cumulative RBC units transfused per patient, cumulative mortality, change in hemoglobin from baseline and time to first transfusion or death. Additional data collected included ICU length of stay, hospital length of stay, and days receiving mechanical ventilation.
A total of 1,302 patients were enrolled in the study; 652 were assigned to the placebo group, and 650 were assigned to the epoetin alpha group. All patients were followed for 28 days after randomization. Patients in the epoetin alpha group were less likely to receive a transfusion compared with the placebo group (50.5% vs 60.4%; P<.001). The cumulative number of units transfused was 19% lower in the epoetin alpha group (P=.04). Hemoglobin increased from baseline in the epoetin alpha group by 1.32 mg/dL versus 0.94 mg/dL in the placebo group (P<.001). There was no difference in mortality, adverse events, length of stay, ventilator-free days, or in any other clinical outcomes between the epoetin alpha and placebo groups.
Limitations to this study included the lack of criteria that mandated RBC transfusions; the lower serum ferritin, transferrin saturation, and serum iron levels in the placebo group at the time of admission; lack of clinical significance of mean difference in increase of 0.38 g/dL in hemoglobin from baseline to study end in the epoetin alpha group versus the placebo group; dose of parenteral iron was not provided; and no clinical outcomes designated as primary end points. Also, the low percentage of total patients screened and enrolled in the study (3.8%) raises questions as to whether the patients who were included were representative of typical ICU patients.
In summary, Corwin et al demonstrated no difference in efficacy in terms of mortality, ICU stay, hospital stay, adverse events, or any other clinical outcome. The only benefit supporting epoetin alpha use in the ICU in both studies was reduction in blood transfusions.
EVALUATION OF SAFETY AND SUPPLY OF RBC TRANSFUSIONS

Other adverse effects associated with blood transfusions, aside from transmission of infectious disease, include: allergic reactions, RBC alloimmunization, and leukocyte/platelet alloimmunization. These adverse effects have minimal clinical consequences in most patients. Acute hemolytic reactions, delayed hemolytic reactions, and transfusion-related acute lung injury are more serious adverse events but occur less frequently.11
RBC transfusions have been theorized to have an immunomodulating effect that increases the risk for bacterial infections and cancer recurrence, but studies have demonstrated inconsistent results regarding these risks.13–17 Leukocyte-reduced blood may decrease the potential for immunosuppression and infection since these risks are thought to be related to the white cell burden.12
RBC supply shortages have been reported but are cyclical and vary from one institution to another. The director of transfusion services at Hartford Hospital indicated that blood supply was not an issue and did not foresee any problems with supply in the near future. However, in general, transfusion practices should be modified to reduce the number of RBC units transfused.
In summary, the overall risk of blood-borne transmission of infection and other adverse events with RBC transfusions is minimal, and issues with blood supply vary from one institution to another. The blood supply at Hartford Hospital was not an issue.
COST CONSIDERATIONS FOR TRANSFUSION REDUCTION
The cost of using epoetin alpha for transfusion reduction in the ICU versus the issues of blood supply and safety, clinical outcomes, and blood conservation techniques are all factors that need to be considered in determining the value of using epoetin alpha in the ICU for this indication. A number of blood conservation techniques to limit blood loss and transfusions have been implemented hospital-wide at Hartford Hospital as a result of the institution's Bloodless Medicine and Surgery Program.
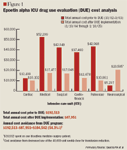
The cost of leukocyte-reduced blood at Hartford Hospital is $215 per unit. Based on the amount of epoetin alpha received by patients in order to provide a 19% reduction in blood transfusions in the study by Corwin et al,7 an expenditure of $112,067 annually on epoetin alpha was incurred to theoretically save $14,349 in blood transfusions in Hartford Hospital's ICUs. This expenditure does not include the cost of IV iron and laboratory tests (ferritin, transferrin saturation) to monitor iron levels.
In summary, we determined that the cost of utilizing epoetin alpha to reduce transfusions in Hartford Hospital's ICUs was high, and the literature reported no benefit in terms of clinical outcomes, including mortality and length of ICU stay. Additionally, the hospital's Bloodless Medicine and Surgery Program had established a number of conservation practices to reduce blood loss and transfusions.
REVIEW OF OTHER CLINICAL ISSUES
It has been reported that the transferrin saturation was below 20% in at least 50% and up to 72% of patients in the ICU.18 Intravenous iron should be administered to these patients who are receiving epoetin alpha to avoid poor utilization (RBC production) of the agent. However, the use of IV iron in the septic and/or immunocompromised ICU patient is controversial due to the potential for increased bacterial virulence. It has been proposed that iron can increase the risk of infection directly by stimulating bacterial growth and indirectly by impairing phagocytosis and impairing host resistance.19 The risk of infection increases when the patient's serum transferrin is low, such as in the malnourished Kwashiorkor patient. Although the relationship between IV iron administration and infection is not definitive, most clinicians would withhold IV iron therapy in ICU patients who have sepsis or who are severely malnourished or immunocompromised.19
It has been demonstrated that the responsiveness to epoetin alpha is reduced in inflammatory states. Even patients without impaired erythropoiesis require at least a week or more to increase the hemoglobin concentration upon initiation of epoetin alpha therapy.20 The majority of transfusions may be required during the first week in the ICU; thus, epoetin alpha therapy may not prevent blood transfusions in a significant number of patients admitted to the ICU.18
Epoetin alpha administration may lead to adverse outcomes, especially in patients at risk for thrombotic events. In a study of coronary bypass patients without renal disease who were randomized to receive epoetin alpha or placebo perioperatively to reduce RBC transfusions, patients in the epoetin alpha group had a higher mortality rate than patients in the placebo group.21 There were 7 deaths in 126 patients randomized to the epoetin alpha group versus 0 deaths among 56 patients receiving placebo (P=.06). Four of the 7 deaths in the treatment group occurred during the period of drug administration, and all 4 of those deaths were associated with thrombotic events. This resulted in a warning by the manufacturers in the package insert stating that in patients at risk for thrombosis, the anticipated benefits of epoetin alpha treatment should be weighed against the potential for increased risks associated with therapy.4,5
RECOMMENDATIONS AND DECISIONS OF THE P&T COMMITTEE
After a review of the previously discussed information, the DIC at Hartford Hospital, in collaboration with the directors of Surgical Critical Care and Transfusion Services, presented the following recommendations to the P&T committee regarding the use of epoetin alpha in the hospital's ICUs:
The above restrictions should not apply to Bloodless Medicine and Surgery Program patients.
The P&T committee approved these recommendations and requested that a DUE be performed to monitor compliance, adverse effects, and cost avoidance.
DUE PROGRAM
The implementation of the DUE involved:
All ICUs were provided with adverse drug reaction (ADR) forms to be completed by caregivers and were instructed to send the forms to the Department of Pharmacy Services if there were any ADRs with blood transfusions.
Once the P&T committee approved the DUE program, we focused on communicating with departments and individuals that would be impacted by the criteria of the program. We obtained the support of the ICU medical directors and caregivers prior to implementation based on the evidence provided in the literature as well as cost considerations. The medical director of the Blood Bank and Transfusion Service also supported the program. We educated the pharmacy staff regarding their role with the program (ie, contacting the prescriber to make a change).
DISCUSSION
Efforts to reduce blood transfusions and the associated risks have been in the forefront of ICU care for more than a decade. Several initiatives have been undertaken to improve the safety of the blood supply and significantly decrease the frequency of blood transfusions in this setting. Very high on the list of initiatives to minimize RBC transfusions in this setting has been the use of epoetin alpha for managing anemia in critical illness.
The recommendation to use epoetin alpha for anemia in critical illness was met with many debates and a divergence of opinions. The "landmark" study by Corwin et al7 in 2002 that utilized 40,000 units of epoetin alpha weekly provided some useful information but left some issues and concerns unanswered. Although iron therapy was used, the dose for IV administration was not provided, the average time to onset of therapeutic effect for epoetin alpha was not reported, the criteria for blood transfusion were not consistent in the study group, and the true clinical benefit of erythropoietin therapy was not determined.
Absolute or functional iron deficiency is a major cause of epoetin alpha therapy failure, and iron deficiency develops quickly after initiation of epoetin alpha as a result of the utilization of large amounts of iron for the production of new erythrocytes.4,5 Oral iron therapy may not provide adequate absorption to replenish or maintain iron stores, according to data in hemodialysis patients. The majority of patients on epoetin alpha are placed on IV iron.7,22 Despite this published information, a significant number of ICU intensivists will not allow the use of IV iron in ICU patients receiving epoetin alpha due to concerns over increased bacterial virulence associated with iron overload.7,8 If IV iron cannot be appropriately administered to ICU patients, epoetin alpha therapy may be ineffective and therefore inappropriate for this patient population.
The average onset of a therapeutic hematopoietic response for epoetin alpha in this specialized population was not reported by Corwin et al.7 If the average onset of this response in ICU patients is about 13 days, as reported by van Iperen at al,9 epoetin alpha therapy may not be a cost-effective modality for most patients in the critical care setting whose needs for transfusions are usually much sooner. Also, critically ill patients who are septic or have an ongoing inflammatory process may have a blunted response to epoetin alpha therapy. Their reticulocyte count increases without a corresponding increase in their hemoglobin.9
RBC transfusions have been viewed as a quick, effective means of improving or providing oxygen delivery to tissues. This practice is very common in ICU patients due to anemia in critical illness. A recent review of transfusion practice in the United States determined that about 90% of transfusions were in part due to low hemoglobin concentrations without any identifiable physiologic reason.23 Also, the hemoglobin concentration that provides for a transfusion threshold in critically ill patients is still controversial. The Transfusion Requirements in Critical Care (TRICC) trial by Hebert et al24 comparing liberal transfusion practice (hemoglobin 10–12 g/dL) to restrictive transfusion practice (hemoglobin 7–9 g/dL) in critically ill patients demonstrated a lower in-hospital mortality in the restrictive transfusion group. This study suggests that a transfusion practice with a lower hemoglobin level of 7 g/dL as a threshold or trigger for transfusion may lead to better clinical outcomes and fewer transfusions.

A follow-up literature search in September 2006 identified 2 studies focusing on transfusion reduction with the use of epoetin alpha in critically ill patients.26,27 Although these studies were published after the implementation of Hartford Hospital's DUE program, they also support our previous conclusion that the only documented benefit for the use of epoetin alpha in critically ill ICU patients is transfusion reduction, with no improved clinical outcomes demonstrated.
The first study, by Silver et al,26 was a prospective, randomized, double-blind, placebo-controlled, multicenter trial that assessed the efficacy of epoetin alpha therapy for transfusion reduction in 86 patients admitted to a long-term acute care facility. As in previous studies, a significant reduction in RBC transfusions in the epoetin alpha group was demonstrated, but there was no difference in clinical outcomes (ie, mortality, serious clinical adverse events) between the treatment and placebo groups.
The second study, by Georgopoulos et al,27 was a prospective, randomized, multicenter trial that assessed the efficacy of 2 dosing schedules of epoetin alpha in increasing hemoglobin and reducing RBC transfusions in 148 patients in 13 ICUs. The 2 epoetin alpha dosing schedules used (40,000 units once a week and 40,000 units 3 times a week) significantly reduced the need for RBC transfusion; however, no clinical outcomes were assessed in this study.
Since the only benefit demonstrated thus far supporting epoetin alpha use in ICU patients is transfusion reduction, further studies in critically ill ICU patients are necessary to determine whether reduction in RBC transfusions will also result in improved clinical outcomes such as decreased mortality, decreased length of stay, or decreased serious clinical adverse events.
CONCLUSIONS
A critical review of the published literature failed to establish the use of epoetin alpha as the standard of care. The cost to reduce RBC transfusions with epoetin alpha in critically ill patients is excessive when there have been no improved clinical outcomes demonstrated to date. The development and implementation of a DUE restricting epoetin alpha use to currently approved FDA indications resulted in substantial cost savings to the institution. Further, supply issues or adverse effects did not limit the use of RBC transfusions when there was a clinical need to increase hemoglobin in critically ill ICU patients.
Editors' Note: This paper is based on a poster that was originally presented at the American College of Clinical Pharmacy (ACCP) Spring Practice and Research Forum, April 10–13, 2005, Myrtle Beach, SC.
Robert A. Quercia, MS, RPh, is clinical manager and director of Drug Information, Department of Pharmacy Services, Hartford Hospital, Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn. He can be reached at rquerci@harthosp.org
. Dr Udeh is clinical coordinator, surgery, Department of Pharmacy Services, Hartford Hospital. Dr Keating is director, Surgical Critical Care, and director, Nutritional Support Service, Department of Surgery, Hartford Hospital, and assistant professor of surgery, University of Connecticut School of Medicine. Dr Sherburne is medical director, Blood Bank and Transfusion Service, Hartford Hospital and Connecticut Children's Medical Center, Hartford, Conn. Dr Goldman is a drug information specialist, Department of Pharmacy Services, Hartford Hospital.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Goodnough LT, Strasburg D, Riddell J 4th, Verbrugge D, Wish J. Has recombinant human erythropoietin therapy minimized red-cell transfusions in hemodialysis patients? Clin Nephrol. 1994;41:303–307.
2. MacDougall IC, Lewis NP, Saunders MJ, et al. Long term cardiorespiratory effects of amelioration of renal anemia by erythropoietin. Lancet. 1990;335:489–493.
3. Mayer G, Thum J, Caca EM, Stummvoll HK, Graf H. Working capacity is increased following recombinant human erythropoietin treatment. Kidney Int. 1988;34:525–528.
4. Procrit [package insert]. Raritan, NJ: Ortho Biotech Products, LP; 2004.
5. Epogen [package insert]. Thousand Oaks, Calif: Amgen Inc; 2006.
6. Corwin HL, Gettinger A, Rodriguez RM, et al. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346–2350.
7. Corwin HL, Gettinger A, Pearl RG, et al; EPO Critical Care Trials Group. Efficacy of recombinant human erythropoietin in critically ill patients. JAMA. 2002;288:2827–2835.
8. Gabriel A, Kozek S, Chiari A, et al. High-dose recombinant human erythropoietin stimulates reticulocyte production in patients with multiple organ dysfunction syndrome. J Trauma. 1998;44:361–367.
9. van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med. 2000;28:2773–2778.
10. Still JM Jr, Belcher K, Law EJ, et al. A double-blinded prospective evaluation of recombinant human erythropoietin in acutely burned patients. J Trauma. 1995;38:233–236.
11. Carson JL. Should patients in intensive care units receive erythropoietin? JAMA. 2002;288:2884–2886.
12. Taylor RW, Manganaro L, O'Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249–2254.
13. Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–1376.
14. Heiss MM, Mempel W, Jauch KW, et al. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet. 1993;342:1328–1333.
15. Jensen LS, Andersen AJ, Christiansen PM, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513–516.
16. Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet. 1996;348:841–845.
17. Houbiers JG, Brand A, van de Watering LM, et al. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;344:573–578.
18. von Ahsen N, Muller C, Serke S, Frei U, Eckardt KU. Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients. Crit Care Med. 1999;27:2630–2639.
19. Kumpf VJ. Update on parenteral iron therapy. Nutr Clin Pract. 2003;18:318–326.
20. Danielson B. R-HuEPO hyperesponsiveness-who and why? Nephrol Dial Transplant. 1995;10(suppl 2):69–73.
21. D'Ambra MN, Gray RJ, Hillman R, et al. Effect of recombinant human erythropoietin on transfusion risk in coronary bypass patients. Ann Thorac Surg. 1997;64:1686–1693.
22. Sunder-Plassmann G, Horl WH. Erythropoietin and iron. Clin Nephrol. 1997;47:141–157.
23. Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study: anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32:39–52.
24. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417.
25. Shermock KM, Horn E, Lipsett PA Pronovost PJ, Dorman T. Number needed to treat and cost of recombinant human erythropoietin to avoid one transfusion-related adverse event in critically ill patients. Crit Care Med. 2005;33:497–503.
26. Silver M, Corwin MJ, Bazan A, Gettinger A, Enny C, Corwin HL. Efficacy of recombinant human erythropoietin in critically ill patients admitted to a long-term acute care facility: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2006;34:2310–2316.
27. Georgopoulos D, Matamis D, Routsi C, et al. Recombinant human erythropoietin therapy in critically ill patients: a dose-response study [ISRCTN48523317]. Crit Care. 2005;9:R508–R15.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
