- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Moderna’s COVID-19 Vaccine Receives Full FDA Approval
FDA is requiring postmarketing surveillance studies to further assess the risk of myocarditis and pericarditis from the vaccine, which is being sold under the brand name Spikevax.
Moderna’s COVID-19 vaccine, sold under the brand name Spikevax received full FDA approval for the prevention of COVID-19 in adults 18 years and older yesterday (Jan. 31).
It is the second COVID-19 to receive full approval. Pfizer-BioNTech’s vaccine was the first. It was approved in August 2021.
“While hundreds of millions of doses of Moderna COVID-19 vaccine have been administered to individuals under emergency use authorization (EUA), we understand that for some individuals, FDA approval of this vaccine may instill additional confidence in making the decision to get vaccinated,” said Janet Woodcock, M.D., acting FDA Commissioner in a press release.
“This is a momentous milestone in Moderna’s history as it is our first product to achieve licensure in the U.S.,” said Stéphane Bancel, Moderna’s CEO, said in a press release.
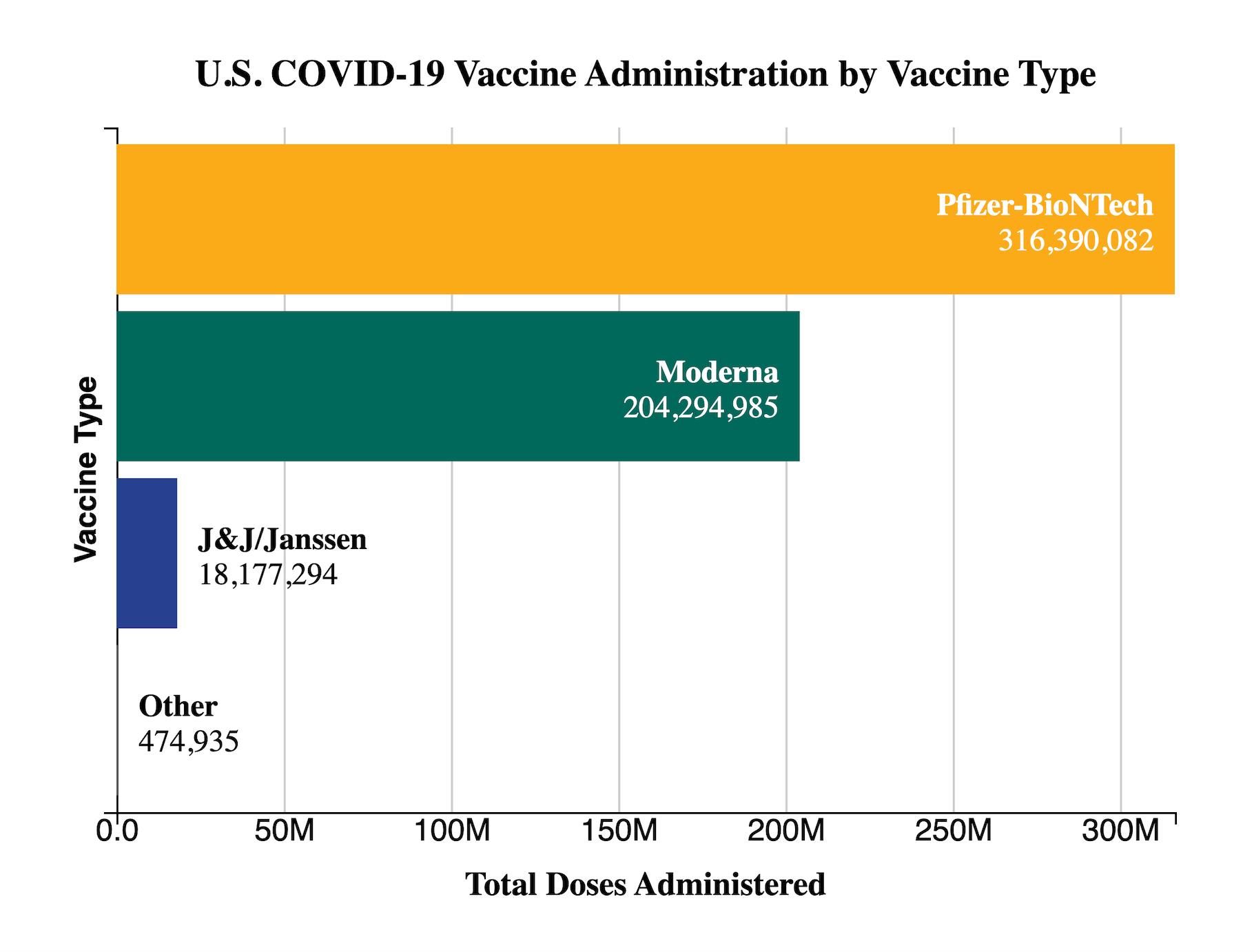
Moderna’s COVID-19 Vaccine has been available under EUA for individuals 18 years and older since December 2020. Approximately 204 million doses have been administered according to the latest CDC data.
Source: CDC
Full approval was granted based on the FDA’s evaluation of safety and efficacy from the ongoing randomized, placebo-controlled Phase 3 COVE study, which included 14,287 patients in the vaccine group and 14,164 study participants in the placebo group. The study demonstrated that Spikevax was 93% effective in preventing COVID-19, with 55 cases occurring in the vaccine group and 744 cases in the placebo group. Additionally, the vaccine was 98% effective at preventing severe COVID-19 disease. The data used for the FDA analysis occurred prior to the omicron variant started to circulate.
The most common reported adverse effects were pain, redness, and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, nausea/vomiting, fever, and swollen lymph nodes under the arm. The prescribing information discusses an increased risk of myocarditis and pericarditis following vaccination, especially seven days after the second vaccine dose in males 18-24 years. Therefore, the FDA is requiring postmarketing surveillance studies to further assess the risk of myocarditis and pericarditis with Spikevax.
Spikevax is administered as a two-dose series intramuscularly 28 days apart, which is considered the primary series. Individuals 18 years and older are eligible for a booster dose five months after completing the primary series based on CDC guidelines.
In a recent CDC study, the effectiveness of a third dose of Moderna and Pfizer-BioNTech vaccines were evaluated in patients who were immunocompetent and in individuals with immunocompromising conditions. The study showed that a third dose — a booster dose — increased vaccine efficacy against hospitalization among adults without and with immunocompromising conditions from 82% to 97% and from 69% to 88% respectively.
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
