- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
New oral anticoagulants: Their impact on efficacy and safety in patients undergoing total hip and total knee replacement and their influence on postoperative management
With the aging of the US population, elective total hip and knee replacement operations have become more prevalent, and costs to the healthcare system for these procedures are increasing rapidly. This includes costs related to treatment of venous thromboembolism, which may be a consequence of these operations if appropriate postoperative thromboprophylaxis is not administered.
Abstract
With the aging of the US population, elective total hip and knee replacement operations have become more prevalent, and costs to the healthcare system for these procedures are increasing rapidly. This includes costs related to treatment of venous thromboembolism, which may be a consequence of these operations if appropriate postoperative thromboprophylaxis is not administered. Among thromboprophylactic agents, the low-molecular-weight heparins, such as enoxaparin, are most commonly used in the hospital setting. Both the low-molecular-weight heparins and warfarin have management problems that may be eliminated by the use of new oral anticoagulants that are either recently approved or in phase 3 development. This article explores evidence for benefits of and potential safety issues associated with the new oral anticoagulants, particularly insofar as these may affect the cost effectiveness of these agents compared with the current standard of care. (Formulary. 2012;47:197–206)
Not surprisingly, given the aging US population, the prevalence of total hip replacement (THR) and total knee replacement (TKR) operations continues to increase as middle-aged and elderly patients seek relief from the pain of debilitating osteoarthritis-the most common joint disorder in the United States-and the opportunity to remain active and maintain their quality of life.1 Concurrently, the number of elective joint replacement operations performed in young patients is also increasing. This trend portends more revision surgeries as those patients grow older. The increasing prevalence of obesity, which is associated with osteoarthritis of both the hip and the knee, also is likely to contribute to the growing rate of THR/TKR operations.1 In 2005, there were more than 800,000 hospital discharges after THR and TKR, but it is estimated that by 2030, the annual number of THR surgeries will reach 572,000, and the number of TKRs will reach 3.48 million.1 Inevitably, this will result in a higher number of failures, which require often-complicated revision surgery. In 2011, authors of a systematic review of international registries reported that the rates of hip and knee revision surgery were similar, at about 12% after 10 years.2
However, none of the known thromboprophylactic agents has a perfect efficacy record in all patients: even with thromboprophylaxis, VTE may occur in up to 10% of patients during the 3-month period after surgery.4 In addition to the enormous burden VTE presents to patients and caregivers, it is a costly condition: a recent study documented that VTE-associated inpatient and outpatient care costs the US healthcare system as much as $39.5 billion annually.5 Using data from a large insurance claims database, a group of investigators documented that charges for patients experiencing either in-hospital or postdischarge VTE after major orthopaedic surgery were much higher compared with charges for patients who did not have VTE.6
Once it occurs, VTE has a high rate of recurrence, with 7% to 14% of patients experiencing a subsequent event.7 In addition, 15% to 50% of patients with a VTE event will develop postthrombotic syndrome within 1 to 2 years after the initial event.7 Without prophylaxis, PE is fatal in about 1 of 300 patients who undergo THR, but approximately 1 in 25 patients with nonfatal PE will, within 2 years, develop chronic thromboembolic pulmonary hypertension.4,8 Costs associated with these complications of VTE may be more than 20% higher than those for the initial event, owing to repeated hospitalizations, long-term therapies, and patient monitoring.9
THROMBOPROPHYLAXIS IN THR/TKR
The American College of Chest Physicians (ACCP) has sponsored publication of guidelines prepared by a multidisciplinary, international group of experts since 1986. The 9th edition of the guidelines, like the previous edition, recommends use of low-molecular-weight heparin (LMWH), fondaparinux, and vitamin K antagonists (VKAs; generally warfarin).4,10 However, unlike the previous edition, the 9th edition states that aspirin is allowed, and it also includes recommendations for one of the new oral anticoagulants (dabigatran, rivaroxaban, or apixaban).
In contradistinction to all previous guidelines, in the 9th edition, conclusions are based on clinically important end points: symptomatic and fatal PE and symptomatic DVT. The studies reported in this present review are based on venographically confirmed DVT, which has been confirmed to be a suitable surrogate for symptomatic events. Venographic DVT allows inclusion of fewer patients in a single study, a matter of importance in phase 2 studies, in which the use of larger numbers may expose some groups to a less-effective dosage regimen and unacceptable risk. By designing the larger phase 3 studies to permit a preplanned pooled analysis of several trials, one can also increase the sensitivity of the analysis of efficacy and base conclusions on the clinically important symptomatic events.
The ACCP guidelines are followed in most hospitals.11 Some protocols call for subcutaneous administration of LMWH (eg, enoxaparin) to all patients undergoing THR and TKR while in the hospital. Postdischarge, patients can continue with injections or transition to an oral agent (most often the VKA warfarin) for a prescribed period (up to 35 days in patients who have undergone THR and at least 10 days in patients who have undergone TKR, although up to 35 days is considered to be beneficial for those patients as well).4 Continued parenteral therapy in an outpatient setting is problematic because it requires either patient education in self-injection, which is not acceptable to all patients, or administration by a caregiver, which is expensive.
Evidence shows, however, that caregivers often do not adhere to published guidelines for anticoagulation.12,13 Although compliance in type, duration, initial timing, and dose was achieved in only 47% of patients who underwent THR and 61% of patients who underwent TKR, the Global Orthopaedic Registry (GLORY) found that almost all patients initially received some form of thromboprophylaxis.12 However, 26% of THR and 27% of patients who underwent TKR were not receiving prophylaxis 7 days after surgery.13 Such nonadherence to published guidelines occurs for a variety of reasons, but a major reason may be the serious management challenges that limit the usefulness of the traditional thromboprophylactic agents. For example, all heparins may lead to heparin-induced thrombocytopenia, necessitating frequent platelet monitoring; in addition, these agents are administered subcutaneously and are thus not convenient to use in an outpatient setting. Because hospital stays after THR/TKR are becoming increasingly shorter, and continued thromboprophylaxis beyond the few days patients spend in hospital is mandated by guidelines, it is important that anticoagulant therapy be simple and comfortable for patients to use postdischarge.
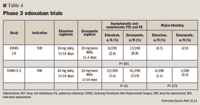
STANDARDS OF CARE
For more than 60 years, the standard of ambulatory care was the anticoagulant warfarin-although today, LMWHs have become the preferred agents for prophylaxis in the acute-care setting in most of the world. Although warfarin's oral formulation makes it more convenient and better suited than injectable agents for outpatient use, it has numerous management challenges that make it problematic for both patients and clinicians. Among these are slow onset and offset of action, a narrow therapeutic window, unpredictable pharmacokinetics and pharmacodynamics, genetic polymorphism, numerous drug-drug interactions, and interactions with many foods containing vitamin K. These factors result in a requirement for routine coagulation monitoring (international normalized ratio [INR]) and frequent dose adjustment to maintain adequate protection while avoiding potentially lethal bleeding events.14-16 Moreover, studies have shown that it is difficult to maintain patients in the recommended therapeutic INR range of 2.0–3.0. In the most meticulously conducted case-control studies, patients have been in therapeutic range only about two-thirds of the time.17 Moreover, when compared with the new oral anticoagulant apixaban for VTE prophylaxis after THR surgery, warfarin was less efficacious. The Apixaban Prophylaxis in Patients undergoing Total Knee Replacement Surgery (APROPOS) study is the only trial in which warfarin was compared with a new oral anticoagulant for safety and efficacy outcomes in patients undergoing TKR.18 In this phase 2 study, 1,238 patients were randomly assigned to apixaban 5 mg, 10 mg, or 20 mg once a day or 2.5 mg, 5 mg, or 20 mg twice a day; enoxaparin 30 mg twice a day; or open-label warfarin titrated to an INR of 1.8 to 3.0. After 10 to 14 days, all apixaban regimens showed relative risk reductions of 21% to 69% compared with enoxaparin and 53% to 82% compared with warfarin. The authors speculate that the low efficacy rate seen with warfarin might have been a reflection of the fact that 49% of the patients were below the target INR range on the day of venography, day 12 ± 2. This, however, demonstrates the difficulty in achieving an ideal INR in the acute-care setting in the context of a well-regulated phase 2 study.
An earlier meta-analysis was conducted to determine the benefit–risk ratio of VKAs relative to comparators.19 An exhaustive literature search was performed, and studies were evaluated according to outcome measures (eg, DVT, PE, death, major hemorrhage, and wound hematoma). Although VKAs were more effective than placebo in reducing DVT and PE, they were less effective than LMWHs in preventing total and proximal DVT, with no significant increase in bleeding. These findings have contributed to warfarin being underprescribed for this indication, as well as to poor patient adherence. Both of these situations leave many patients unprotected or suboptimally protected against VTE, with a consequent excess of clinical events.
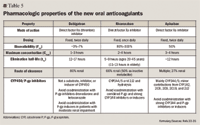
NEW ORAL ANTICOAGULANTS
DIRECT THROMBIN INHIBITOR: DABIGATRAN
Dabigatran is a reversible direct inhibitor of both free and clot-bound thrombin.33 Its reversibility allows the possibility of some residual thrombin being available for clotting.
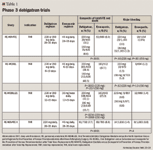
Dabigatran etexilate has been studied for the prevention of VTE in the Dabigatran Etexilate versus Enoxaparin for Prevention of Venous Thromboembolism after Total Hip Replacement (RE-NOVATE) trial, the Oral Dabigatran versus Subcutaneous Enoxaparin for the Prevention of Venous Thromboembolism after Total Knee Replacement trial (RE-MODEL), and the Oral Thrombin Inhibitor Dabigatran Etexilate versus the North American Enoxaparin Regimen for the Prevention of Venous Thromboembolism after Knee Arthroplasty Surgery trial (RE-MOBILIZE).20–22
In all of the studies, the primary efficacy end point was the composite of total VTE and all-cause death. RE-NOVATE was designed to compare the efficacy and safety of 2 doses of dabigatran etexilate (220 mg daily [N=1,157] or 150 mg daily [N=1,174]) with the LMWH enoxaparin 40 mg once daily starting the evening before surgery (N=1,163) for a 28- to 35-day period (median duration, 33 days) in patients undergoing THR.20 Both doses of dabigatran etexilate were started 1 to 4 hours postoperatively at half the dose (110 mg or 75 mg). The primary efficacy outcome occurred in 60 patients (6.7%) in the enoxaparin group versus 53 patients (6.0%) in the dabigatran etexilate 220-mg group (absolute difference: –0.7%; 95% confidence interval [CI], 2.9%–1.6%) and 75 patients (8.6%) in the dabigatran etexilate 150-mg group (absolute difference, 1.9%; 95% CI, –0.6% to 4.4%). Thus, both dabigatran etexilate doses were noninferior to enoxaparin. The incidence of major bleeding events was low (~1.5%); there was no significant difference in the frequency of major bleeding events during treatment among both of the dabigatran etexilate doses and enoxaparin (P=.44 for 220 mg and P=.60 for 150 mg, respectively); and both doses of dabigatran etexilate had a similar adverse event profile to that of enoxaparin.
Similar to RE-NOVATE, in the RE-MODEL trial, the 2,101 patients undergoing TKR were randomly assigned to receive dabigatran etexilate 220 mg daily, dabigatran etexilate 150 mg daily, or enoxaparin 40 mg daily, this time for 6 to 10 days, with a follow-up period of 3 months.21 The primary efficacy outcome in RE-MODEL, a composite of total VTE and mortality during treatment, occurred in 36.4% of the dabigatran 220-mg group, 40.5% of the dabigatran etexilate 150-mg group, and 37.7% of the enoxaparin group (absolute difference vs enoxaparin, –1.3% for the dabigatran 220-mg group and +2.8% for the 150-mg group; 95% CI, –7.3 to 4.6 and –3.1 to 8.7, respectively). During treatment, major bleeding events occurred in 1.5% of patients (95% CI, 0.7–2.7) in the dabigatran 220-mg group; 1.3% of patients (95% CI, 0.6–2.4) in the dabigatran 150-mg group; and 1.3% of patients (95% CI, 0.6–2.4) in the enoxaparin group. Differences in bleeding events among either dose of dabigatran etexilate and enoxaparin were not significant (P=.82 for 220 mg and P=1.0 for 150 mg). Adverse events leading to treatment discontinuation occurred in 3.7% of both dabigatran etexilate groups and in 4.6% of the enoxaparin group.
Although in both RE-NOVATE and RE-MODEL, dabigatran demonstrated noninferiority to enoxaparin, in RE-MOBILIZE, dabigatran failed to meet the noninferiority criteria compared with enoxaparin in patients undergoing TKR.22 In this trial, 2,615 patients were randomized to treatment with dabigatran etexilate at doses of 220 mg or 150 mg daily or enoxaparin 30 mg twice a day for 12 to 15 days after surgery (median, 14 days). In RE-MOBILIZE, dabigatran etexilate was started 6 to 12 hours postoperatively at half dose, and enoxaparin was started 12 to 24 hours postoperatively. The primary efficacy outcome was total VTE and death, and 1,896 patients were included in the primary efficacy analysis. Results showed both doses of dabigatran to have inferior efficacy compared with enoxaparin, with VTE rates of 31% (P=.02 vs enoxaparin); 34% (P<.001 vs enoxaparin); and 25%, respectively. Major bleeding was uncommon, and rates were similar among the 3 groups (0.6% for dabigatran 220 mg, 0.6% for 150 mg, and 1.4% for enoxaparin). Investigators hypothesized that the superior efficacy results documented with enoxaparin in this study were attributable to the fact that daily doses were 50% higher than in the RE-MODEL study, doses were administered for 5 days longer, and the test drug was started later.
The RE-NOVATE II trial further evaluated the efficacy and safety of the 220-mg dose of dabigatran compared with enoxaparin 40 mg once daily in a THR population (N=2,055) for 28 to 35 days after surgery.23 The primary efficacy outcome, the composite of total VTE and all-cause mortality during the treatment period, occurred in 7.7% of dabigatran-treated patients versus 8.8% of those treated with enoxaparin; P<.0001 for the prespecified noninferiority margin. Major bleeding occurred in 1.4% of the dabigatran group and 0.9% of the enoxaparin group (P=.40). The incidence of adverse events was similar between the groups. Thus, dabigatran was shown to be at least equivalent to traditional anticoagulant agents for thromboprophylaxis after THR and TKR.
DIRECT FACTOR XA INHIBITORS
These agents selectively and reversibly block free and clot-bound factor Xa and prothrombinase activity and do not require a cofactor such as antithrombin III for their activity.34,35
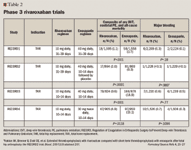
RECORD2 randomly assigned 2,509 patients undergoing THR to receive rivaroxaban 10 mg daily for 31 to 39 days or enoxaparin 40 mg daily for 10 to 14 days and then an oral placebo.25 In the modified intention-to-treat population (N=1,733), the primary efficacy outcome, the composite of symptomatic and venographic DVT, nonfatal PE, and all-cause mortality up to days 30 to 42, occurred in significantly fewer patients receiving extended thromboprophylaxis with rivaroxaban (17 patients; 2.0%) than in those receiving short-term enoxaparin (81 patients; 9.3%) (absolute RR, 7.3%; 95% CI, 5.2–9.4; P<.0001). The incidence of on-treatment bleeding was similar in both groups.
The RECORD3 and 4 trials investigated the efficacy and safety of riva-roxaban versus enoxaparin in patients undergoing TKR.26,27 In RECORD3, 2,531 patients were randomly assigned to receive either 10 mg of rivaroxaban daily or 40 mg of enoxaparin daily starting preoperatively for a maximum of 14 days.26 The primary efficacy outcome, the composite of all DVT, nonfatal PE, or death from any cause 13 to 17 days after surgery, occurred in 79 patients (9.6%) in the rivaroxaban group and in 166 patients (18.9%) in the enoxaparin group (absolute RR, 9.2%; 95% CI, 5.9–12.4; P<.001). Major bleeding occurred in 7 of 1,220 patients (0.6%) who received rivaroxaban and in 6 of 1,239 patients (0.5%) who received enoxaparin, and there were similar adverse events in the 2 groups. RECORD4 randomly assigned 3,148 patients undergoing TKR to either rivaroxaban 10 mg daily or enoxaparin 30 mg twice a day starting the day after surgery.27 The primary efficacy outcome, the composite of any DVT, nonfatal PE, or death from any cause up to 17 days after surgery, occurred in 67 (6.9%) of the 965 patients receiving rivaroxaban and in 97 (10.1%)-significantly more-of the 959 patients receiving enoxaparin (absolute RR, 3.19%, 95% CI, 0.71–5.67; P=.0118). In RECORD4, 0.7% of patients given rivaroxaban and 0.3% of patients given enoxaparin had major bleeding (P=.1096).
This trial was the only study in the RECORD program to use the North American dosing regimen for enoxaparin, which is higher than that used in the RECORD1–3 trials. None of the other new oral anticoagulants achieved noninferiority against this regimen. In addition, in RECORD4, enoxaparin was started 12 to 24 hours after wound closure, which was later than in the other trials, although a twice-a-day regimen would be expected to produce a more rapid therapeutic response.
Based on the results of RECORD1–3, which showed rivaroxaban to be more effective than enoxaparin for thromboprophylaxis after both THR and TKR, rivaroxaban received FDA approval in 2011 for thromboprophylaxis after THR and TKR. Thus, rivaroxaban was the first of the new oral agents to be approved for this indication in the US.34
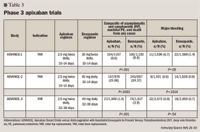
Like ADVANCE-1, the ADVANCE-2 trial was designed to assess the efficacy and safety of apixaban compared with enoxaparin in patients undergoing TKR.29 In ADVANCE-2, 3,057 patients were randomly assigned to receive apixaban 2.5 mg twice a day or enoxaparin 40 mg daily, with both drugs continued for 14 days. The primary outcome was the composite of asymptomatic and symptomatic DVT, nonfatal PE, and all-cause death during treatment. This outcome was reported in 15% of patients in the apixaban group, compared with 24% of patients in the enoxaparin group (relative risk, 0.62; 95% CI, 0.51–0.74; P<.0001). Major or clinically relevant nonmajor bleeding occurred in 4% of patients in the apixaban group versus 5% of patients in the enoxaparin group (P=.09).
ADVANCE-3 randomly assigned 5,407 patients to receive either apixaban 2.5 mg twice a day or enoxaparin 40 mg daily for 35 days after THR; patients were followed-up for an additional 60 days after the last dose of study medication.30 The primary efficacy outcome was the composite of asymptomatic or symptomatic DVT, nonfatal PE, or death from any cause during the treatment period. Of the 1,949 assessable apixaban-treated patients and the 1,917 assessable enoxaparin-treated patients, the primary efficacy outcome occurred in 1.4% and 3.9%, respectively (relative risk with apixaban, 0.36; 95% CI, 0.22–0.54; P<.001 for both noninferiority and superiority). Of the 2,673 patients receiving apixaban and the 2,659 receiving enoxaparin, the composite safety outcome of major and clinically relevant nonmajor bleeding occurred in 4.8% and 5.0%, respectively (absolute difference in risk, –0.2 percentage points; 95% CI, –1.4 to 1.0). Thus, although apixaban was shown to be more effective than enoxaparin for thromboprophylaxis after THR, it showed mixed results after TKR.
SAFETY ISSUES
The risk of bleeding is not limited to the new oral anticoagulants. Bleeding is a risk with all anticoagulants; with LMWH, the risk is 3% to 5% after THR/TKR.4 With the new oral anticoagulants, it is important to be aware of their pharmaceutical properties. The drugs are metabolized in different ways. Thus, dabigatran is 80% renally excreted, whereas apixaban is only 27% renally excreted.33,35 With the former agent, the level of renal function is particularly important. The new oral anticoagulants should be used with caution (at lower doses) in patients with kidney impairment. Dabigatran requires a dose adjustment if the creatinine clearance (CrCl) is less than 30 mL/min, whereas rivaroxaban is not recommended as VTE prophylaxis in patients with a CrCl less than 30 mL/min.33,34 FDA recently undertook an investigation of postmarketing reports of serious bleeding in patients taking dabigatran. Both FDA and the European Medicines Agency have issued advisories on its use in high-risk situations. Hemorrhaging appears to be associated with prolonged use in patients with impaired kidney function or elderly patients with declining renal function.
COST ISSUES
Potential savings associated with the new oral anticoagulants are based on reductions in the incidence of symptomatic VTE events, as well as reductions in administration and monitoring costs. The fact that no routine coagulation monitoring is required with these agents is a major cost saver. In 2000, coagulation monitoring with warfarin was estimated to cost $51.25 per patient per month ($67 in 2011 dollars).40 Despite its subcutaneous route of administration, enoxaparin has been shown to be more cost effective for thromboprophylaxis than warfarin, particularly for long-term use and taking into account long-term complications of VTE.41 However, the fact that enoxaparin is administered subcutaneously adds to its cost; data suggest that, in 2002, a home visit for injection after hospital discharge cost approximately $100 for a single course of enoxaparin.42
For 2 of the new oral anticoagulants, pharmacoeconomic models have been developed, in which the oral anticoagulants have been shown to be more cost effective than enoxaparin. In a 2008 probabilistic sensitivity analysis of the cost effectiveness of dabigatran versus enoxaparin in the British Health Service, it was found that although the efficacy and safety profiles of the 2 agents were similar, modest cost savings were documented with the use of dabigatran in patients undergoing TKR (£137 for dabigatran versus £237 for enoxaparin).43 For patients undergoing THR, for whom extended prophylaxis (up to 7 weeks) is recommended by practice guidelines, estimated cost savings were more substantial. In addition to avoiding the need to train patients in self-administration of an injectable agent, the authors calculated the cost savings when platelet monitoring for heparin-induced thrombocytopenia, needlestick injuries, and needle disposal were avoided with use of the oral agent.
Cost-effectiveness analyses of the RECORD trials have shown significant cost savings with use of rivaroxaban in patients undergoing THR (RECORD1 and 2) compared with enoxaparin, driven mainly by reduced costs of administration and hospitalization associated with fewer VTE events.44,45 In patients undergoing TKR (RECORD3), rivaroxaban was still more cost effective than enoxaparin, if costs of home nurse time or training in self-administration of enoxaparin were included.46 Duran et al. used data from RECORD1–3 in a decision-analytic model to compare outcomes and costs associated with rivaroxaban versus enoxaparin treatment in patients undergoing THR and TKR. The investigators demonstrated that rivaroxaban reduced total treatment payer costs compared with enoxaparin for the prevention of VTE associated with both THR and TKR.47
When determining cost effectiveness, the costs of potential bleeding events must be taken into consideration. Although major bleeding requires physician treatment and possibly emergency department visits or hospitalization, which are expensive, in a retrospective analysis of data on 119,729 patients from a large managed care database during the years 2004–2008, the 3-month risk of any bleed was lower (4%) than the risk of VTE (6.7%). The risk of a major bleeding event was only 1.9%.48 In that analysis, cumulative all-cause healthcare costs were $292,690,000 for the 7,974 patients who had a VTE event, $183,254,000 for the 4,849 patients who had a bleeding event, and $93,570,000 for the subset of patients who had a major bleeding event.
CONCLUSIONS
VTE after THR/TKR contributes significantly to postoperative morbidity, mortality, and healthcare costs. Pharmacologic thromboprophylaxis remains the standard of care. However, the traditionally used agents (warfarin, LMWHs, and fondaparinux) all are associated with potential practical management difficulties. Suboptimal caregiver adherence to published guidelines emphasizes the need for agents that are easier to administer and manage from both patient and caregiver perspectives. The new oral anticoagulants may provide a promising alternative to the traditional agents, and their ease of use and management could potentially lead to improved caregiver adherence to published guidelines, better patient medication adherence, and improved outcomes after THR/TKR. Furthermore, many of the phase 3 studies demonstrated increased efficacy compared with LMWHs, resulting in further economies in the prevention and management of VTE complications that may occur even with effective VTE prophylaxis. In addition, these new agents may result in cost savings for the healthcare system. Further research, including postmarketing research, is warranted.
Dr Fisher is Attending Surgeon, Department of Orthopaedic Surgery, McGill University Health Centre, Montréal Canada.
Disclosure Information: The author reports the following relationships: Bayer Healthcare – Steering Committee member, Principal Investigator, and consultant; sanofi aventis – Steering Committee member, Principal Investigator, and presenter; Bristol-Myers Squibb – Principal Investigator and consultant; Boehringer Ingelheim – Speakers' Bureau and consultant.
REFERENCES
1. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90:1598–1605.
2. Labek P, Thaler M, Jander W, Agreiter M, Stöckl B. Revision rates after total joint replacement: cumulative results from worldwide joint register datasets. J Bone Joint Surg Br. 2011;93:293–297.
3. Maynard G, Stein J. Preventing hospital-acquired venous thromboembolism: a guide for effective quality improvement. Prepared by the Society of Hospital Medicine. AHRQ publication no. 08-0075. Rockville, MD: Agency for Healthcare Research and Quality;2008. Available at http://www.ahrq.gov/qual/vtguide/vtguide.pdf Accessed April 21, 2012.
4. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:381S–453S.
5. Mahan CE, Holdsworth MT, Welch SM, Borrego M, Spyropoulos AC. Deep-vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
6. Oster G, Ollendorf DA, Vera-Llonch M, Hagiwara M, Berger A, Edelsberg J. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother. 2004;38:377–382.
7. MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm. 2006;63(suppl 5):S5–S15.
8. Pengo V, Lensing AWA, Prins MH, et al; for the Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264.
9. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486.
10. Falck-Ytter Y, Francis CW, Johanson N, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e278S–e3255S.
11. Markel DC, York S, Liston MJ Jr, et al. Venous thromboembolism: management by American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25:3–9.
12. Friedman R.J., Gallus AS, Cushner FD, Fitzgerald G, Anderson FA Jr; for the Global Orthopaedic Registry Investigators. Physician compliance with guidelines for deep-vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin. 2008;24:87–97.
13. Warwick D, Friedman RJ, Agnelli G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br. 2007;89:799–807.
14. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:160S–198S.
15. Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:287S–310S.
16. Weitz JI, Hirsh J, Samama MM. New antithrombotic drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:234S–256S.
17. Wallentin L, Yusuf S, Ezekowitz MD, et al; for the RE-LY Investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983.
18. Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost. 2007;5:2368–2375.
19. Mismetti P, Laporte S, Zufferey P, Epinat M, Decousus H, Cucherat M. Prevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: a meta-analysis. J Thromb Haemost. 2004;2:1058–1070.
20. Eriksson BI, Dahl OE, Rosencher N, et al; for the RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956.
21. Eriksson BI, Dahl OE, Rosencher N, et al; for the RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–2185.
22. RE-MOBILZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9.
23. Eriksson BI, Dahl OE, Huo MH, et al; for the RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105:721–729.
24. Eriksson BI, Borris LC, Friedman RJ, et al; for the RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
25. Kakkar AK, Brenner B, Dahl OE, et al; for the RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–39.
26. Lassen MR, Ageno W, Borris LC, et al; for the RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786.
27. Turpie AGG, Lassen MR, Davidson BL, et al; for the RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680.
28. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
29. Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815.
30. Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; for the ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498.
31. Fuji T, Fujita S, Tachibana S, Kawai Y, Koretsune Y, Yamashita T, Nakamura M. Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS J-V trial. Presented at American Society of Hematology Annual Meeting; December 7, 2010; Orlando, FL.
32. Fuji T, Wang C-J, Fujita S, et al. Edoxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty: The Stars E-3 Trial [abstract OC297]. Presented at the 21st International Congress of Thrombosis; July 6-9, 2010; Milan, Italy.
33. PRADAXA (dabigatran etexilate mesylate) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. August 2011.
34. XARELTO (rivaroxaban) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. July 2011.
35. Eliquis (apixaban) summary of product characteristics. Uxbridge, UK: Bristol-Myers Squibb/Pfizer EEIG. May 2011.
36. Perzborn E, Harwardt M. Recombinant factor VIIa partially reverses the effects of the factor Xa inhibitor rivaroxaban on thrombin generation, but not the effects of thrombin inhibitors, in vitro [abstract]. J Throm Haemost. 2007;5(suppl 2):abstract P-W-640.
37. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;[published online ahead of print September 6, 2011].
38. Gruber A, Marzec UM, Buetehorn U, Hanson S, Perzborn E. Potential of activated prothrombin complex concentrate and activated Factor VII to reverse the anticoagulant effects of rivaroxaban in primates [poster]. Blood. 2008[ASH Annual Meeting Abstracts];112:abstract 3825.
39. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127.
40. Anderson RJ. Cost analysis of a managed care decentralized outpatient pharmacy anticoagulation service. J Manag Care Pharm. 2004;10:159–165.
41. Botteman MF, Caprini J, Stephens JM, et al. Results of an economic model to assess the cost-effectiveness of enoxaparin, a low-molecular-weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long-term complications in total hip replacement surgery in the United States. Clin Ther. 2002;24:1960–1986.
42. de Lissovoy G, Subedi P. Economic evaluation of enoxaparin as prophylaxis against venous thromboembolism in seriously ill medical patients: a US perspective. Am J Manag Care. 2002;8:1082–1088.
43. Wolowacz SE, Roskell NS, Maciver F, et al. Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism after total knee and hip replacement surgery. Clin Ther. 2009;31:194–212.
44. Friedman RJ, Lees M, Sengupta N, Haas S. Rivaroxaban for prevention of venous thromboembolism after total hip replacement: impact on healthcare costs based on the RECORD1 study [abstract]. J Bone Joint Surg Br. 2010;92(suppl II):289.
45. Kakkar A, Lees M, Sengupta N, Muntz J. Prevention of venous thromboembolism with rivaroxaban after total hip replacement: economic impact of extended thromboprophylaxis [abstract]. J Bone Joint Surg Br. 2010;92(suppl II):273.
46. Kwong LM, Lees M, Sengupta N. Rivaroxaban for prevention of venous thromboembolism after total knee arthroplasty: impact on healthcare costs based on the RECORD3 study [poster]. Blood. 2007[ASH Annual Meeting Abstracts];110:abstract 1874.
47. Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost and outcomes associated with rivaroxaban vs enoxaparin for the prevention of postsurgical venous thromboembolism from a US payer's perspective. J Med Econ. 2011;14:824–834.
48. Vekeman F, Lamori JC, Laliberte F, et al. Risks and cost burden of venous thromboembolism and bleeding for patients undergoing total hip or knee replacement in a managed-care population. J Med Econ. 2011;14:324–334.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
