- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
New Parkinson's disease pipeline needs to offer improved quality of life, balanced with cost containment
Parkinson's disease, which decreases the amount of dopamine in the brain, affects about 1 million Americans. Available medical therapy cannot modify the progression of the disease; medications have only been shown to alleviate its symptoms.

Key Points
Parkinson's disease (PD), which decreases the amount of dopamine in the brain, affects about 1 million Americans. Available medical therapy cannot modify the progression of the disease; medications have only been shown to alleviate its symptoms.
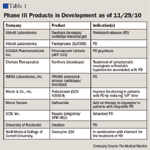
Late-stage clinical trials include compounds that increase dopamine levels or target dopamine or serotonin receptors (IPX066, pardoprunox, and safinamide), and new dosage forms of existing agents (rotigotine transdermal patch [Neupro] and levodopa/carbidopa intestinal gel [Duodopa]). Clinical investigation continues on available over-the-counter (OTC) products, such as coenzyme Q10 and creatine, which may result in clinical benefits based on their antioxidant effects.
COMMENTARY: A MANAGED CARE PERSPECTIVE
There is no cure yet for Parkinson's disease (PD), but modern medicine has changed a 10-year death sentence into a manageable chronic condition with a near-normal life expectancy. Skillful clinicians have a variety of established treatments available, some of which are generic and very cost-effective. However, disease complications and medication side effects accumulate as time passes for those with PD.
From a managed care perspective, this is a smaller-impact condition-less consequential in younger Medicaid and employed populations, but gaining importance in Medicare recipients and retirees. Nevertheless, in the current healthcare environment, no managed care organization (MCO) will ignore reasonable cost-containment efforts, and the new pipeline products afford both promise and reasons for caution and skepticism.
First, the rotigotine patch, which has been reformulated successfully, holds great promise for late-stage PD as adjunctive treatment, and it may find a place as earlier monotherapy as well. Depending on pricing, this product could gain initial placement on formularies-either when prescribed by neurologists or as step therapy after failure of levodopa/carbidopa. Eventual unrestricted access, although with brand copays, may be in the cards.
New oral formulations of extended-release levodopa/carbidopa, such as IPX066, will have to show more meaningful benefits than prolonged improvements in walking speed for MCOs to place this product on formularies at much higher branded costs. The appetite of payers to fund surgically implanted intestinal gel delivery systems (e.g., Duodopa) for levodopa/carbidopa will be minimal except in the most extreme and refractory cases. This kind of therapy would also initially have to go through the medical benefit side, owing to the need for a short procedure, but it would then likely be managed by specialty vendors on an ongoing basis, if approved at all. The costs of potential complications would need to be considered as well.
Coenzyme Q10 is a curious compound with a growing number of possible applications, including in the cardiovascular realm. If the findings of early PD studies bear out in subsequent research, this agent may force MCOs to begin including some OTC products as part of their pharmacy benefit structures. Although not difficult in the Medicaid world, where many OTC products are already covered, this will be a bigger stretch on the commercial side. Initial monotherapy for early PD could be a possibility with coenzyme Q10. This could also be the case for creatine if study outcomes are impressive.
The other pharmacologic products in the pipeline, such as pardoprunox, pimavanserin tartrate, and safinamide, will need to demonstrate both meaningful improvements in quality of life and lower costs from complications, emergency-room visits, and hospitalizations. As in the case of safinamide, gaining an extra 30 minutes of "on" time per day in exchange for the cost of a new branded agent will not gain formulary approvals.
Finally, the future prospect of PD cure via stem cell restorative treatments is intriguing, and holds theoretical potential, but could be short-circuited by ethical considerations, depending upon the sources of those stem cells.
-Lawrence E. Kay, MD, vice president and chief medical officer, Physicians Plus Insurance Corporation, Madison, WI
Copyright 2010 by The Medical Pipeline, LLC. All rights reserved. For more information about The Medical Pipeline, please visit http://www.tmp-pipeline.com/.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
