- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
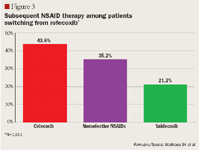
NSAID utilization patterns following market withdrawal of rofecoxib
Cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drugs (NSAIDs) have been widely prescribed for patients with arthritis and other conditions because of their lower risk for gastrointestinal adverse events compared with nonselective NSAIDs 1,2
Cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drugs (NSAIDs) have been widely prescribed for patients with arthritis and other conditions because of their lower risk for gastrointestinal adverse events compared with nonselective NSAIDs.1,2
On September 30, 2004, the COX-2 inhibitor rofecoxib was withdrawn from the market after a clinical trial demonstrated an increased risk of cardiovascular events in patients receiving chronic therapy with the drug.3 At the time of withdrawal, little data were available regarding the cardiovascular safety of other COX-2-selective and nonselective NSAIDs.
OBJECTIVES
STUDY METHODS
The study methods included:
Design: retrospective analysis of electronic pharmacy claims from a large managed care organization in the United States.
Time frame: index date defined as September 30, 2004, with a 4-month pre-index period and a 4-month post-index period.
Patient identification: adult patients aged 18 years or older at index who were continuously enrolled during the pre-index and post-index periods and who had pharmacy claim(s) for a COX-2 inhibitor or nonselective NSAID totaling at least a 90-day supply during the pre-index period.
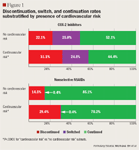
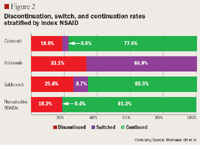
Cohorts: patients were stratified by index NSAID (defined by the last NSAID filled during the pre-index period) into 2 cohorts (COX-2 inhibitor or nonselective NSAID), and further substratified based on the presence of cardiovascular risk, which was defined by a prescription fill for a cardiovascular or diabetes medication.
Baseline measures: demographics; concomitant use of a gastrointestinal-protective agent during the pre-index period; presence of cardiovascular risk.
Follow-up period outcome measures: continuation rate; discontinuation rate; switch rate (classified as switched when the first filled NSAID during the post-index period differed from the index NSAID).
Statistical methods: continuous variables were compared using t-test; categorical variables were compared using chi-square test.
RESULTS

DISCUSSION
CONCLUSIONS
Editors' Note: This study was originally presented as a poster at the Academy of Managed Care Pharmacy (AMCP) 17th Annual Meeting & Showcase, April 20–23, 2005, in Denver, Colo.
Dr Morikawa is a managed care pharmacy resident at Prescription Solutions, Costa Mesa, Calif. He can be reached via email at information@rxsolutions.comMs Vanderplas is a statistician, Dr Chang is chief statistician, and Dr Lew is a researcher with Health Informatics and Outcomes Research at Prescription Solutions. Prescription Solutions is a leading provider of pharmacy and medical management services, managing the prescription drug benefits of commercial, Medicare, and government health plans, employers, and unions, nationwide.
REFERENCES
1. Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR study group. N Engl J Med. 2000;343:1520–1528.
2. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284: 1247–1255.
3. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
