- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Patient access to orphan drugs faces new set of challenges
In 1983, the United States enacted the Orphan Drug Act (ODA). An analogous law was passed in Europe in 2000. Both pieces of legislation are considered major successes in terms of spurring the development of orphan drugs. To illustrate, in the decade prior to 1983 only 34 orphan products were marketed, whereas in the past year alone 9 orphan drugs were launched. In the past 5 years, 39 orphan drugs were launched in the US across numerous therapeutic categories, including multiple myeloma, chronic myeloid leukemia, metastatic non-small cell lung cancer, hemophilia, tuberculosis, homozygous familial hypercholesterolemia, and cystic fibrosis.1
In 1983, the United States enacted the Orphan Drug Act (ODA). An analogous law was passed in Europe in 2000. Both pieces of legislation are considered major successes in terms of spurring the development of orphan drugs. To illustrate, in the decade prior to 1983 only 34 orphan products were marketed, whereas in the past year alone 9 orphan drugs were launched. In the past 5 years, 39 orphan drugs were launched in the US across numerous therapeutic categories, including multiple myeloma, chronic myeloid leukemia, metastatic non-small cell lung cancer, hemophilia, tuberculosis, homozygous familial hypercholesterolemia, and cystic fibrosis.1
Nearly a third of post-2001 orphan approvals generated annual sales of more than $1 billion for at least 1 year in their life cycle. At 8% annually, growth in orphan drugs is twice that of the overall prescription drug market. Furthermore, spending on orphan drugs currently makes up approximately 6% of total pharmaceutical sales, up from under 1% in 2001.2 Orphan products run the gamut from blockbusters with over $7 billion in annual sales such as Rituxan (rituximab) and Gleevec (imatinib mesylate), to niche orphans such as Kalydeco (ivacaftor) and Xalkori (crizotinib) with small subpopulations and correspondingly lower total revenue (Table 1).2,3
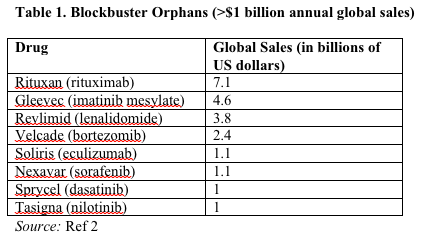
The growing numbers of orphan drugs in the market and the high cost of certain orphan treatments have led to a reconsideration of payer reimbursement policies.4 We examined patient access to orphan drugs approved from 1983 through 2013, analyzing differences in US and European approvals as well as payer reimbursement policies with respect to orphan drugs in the US and 4 leading markets in the European Union: France, Germany, the Netherlands, and the United Kingdom.
First, we tallied regulatory approvals of orphan drugs. For our analysis, we excluded products withdrawn from the market, as well as blood products, vaccines, new indications, and new formulations. Subsequently, we analyzed patient access to orphan drugs in the US and Europe by reviewing 20 leading Medicare payers as well as pricing and reimbursement decisions by health authorities in 4 European countries.
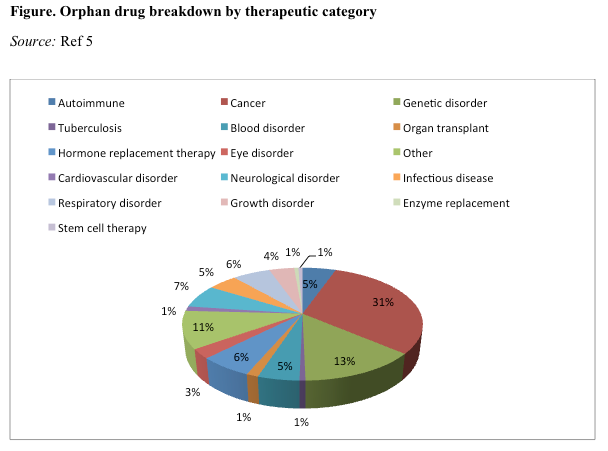
Cancer is the most prevalent therapeutic category and includes orphan drugs targeting multiple myeloma, chronic myeloid leukemia, and brain cancer. Of the 151 orphan drugs approved from 1983 through 2013, drugs to treat cancer dominated with 46 approvals (30%), followed by approvals to treat genetic disorders (20%) and neurologic conditions (10%). Approximately 20% of drugs approved from 1983 through 2013 targeted ultra-orphan diseases, affecting fewer than 1 in 60,000 patients (Figure).5
Joshua P. Cohen, PhD, Tufts Center for the Study of Drug Development, discusses the growing number of orphan drugs in the market as well as the high cost of certain treatments.
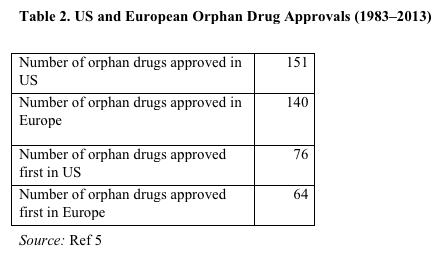
Since enactment of the ODA in 1983, 7% more orphan drugs have been approved in the US than in Europe. In addition, since 1983, 17% more orphan drugs were approved first in the US than were approved first in Europe (Table 2).5
Ten years ago the conventional wisdom among policymakers was that because orphan drugs target small populations their impact on the pharmacy budget was fairly limited. Currently, payers look at orphans differently in light of more approvals, expansion of indications, and higher prices. There is a trend toward much higher patient cost sharing, more imposition of conditions of reimbursement, and in a few cases outright formulary exclusions. Exclusions generally did not happen 10 years ago. Patient cost sharing was much lower and often a fixed copayment rather than coinsurance (percentage of cost to payer). In addition, there was virtually no imposition of step therapy or quantity limits and only minimal use of prior authorization.
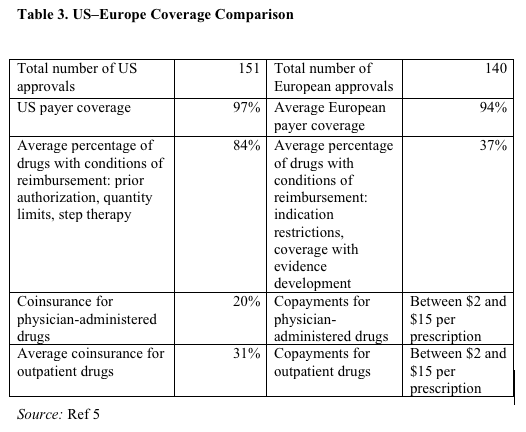
Table 3 shows differences between the US and Europe in terms of orphan drug coverage.5 There are relatively few outright denials of coverage, although more in Europe. There is much higher patient cost sharing in the US. In Europe, a lower percentage of orphan products have conditions of reimbursement; however, the conditions are more stringent. Indication restrictions and coverage with evidence development limit coverage to a subset of approved indications. There is no use of indication restrictions or coverage with evidence development by US payers.
European health authorities have formally evaluated between 5% and 50% of new orphan drug approvals, depending on the jurisdiction. The UK health authority evaluated 25% of orphan drugs; the health authorities in France and the Netherlands 50%; and the German health authority 5%.6 Formal assessments are expected to increase in the near future, especially in the UK and Germany. US payers do not yet systematically conduct a health technology assessment (HTA), but US payers intend to conduct more HTA evaluations.7
Policy implications
The numbers of new orphan approvals point to the success of orphan drug legislation. Moreover, orphan drugs are lucrative for the biopharmaceutical industry.8 There are more of them each year, and at higher prices. Growing costs, however, are leading to a reconsideration of payer reimbursement policies. Thus far the pricing controversy has concerned non-orphans. Policymakers, media, and payers have focused on the cost of non-orphans such as Sovaldi (sofosbuvir) and Zaltrap (aflibercept). In the case of Sovaldi, UnitedHealth has requested pharmacoeconomic data to stratify the hepatitis C population into those who have an immediate need for Sovaldi treatment and those who could delay or even avoid Sovaldi treatment altogether.9 In the case of Zaltrap, in October 2012, oncologists at Memorial Sloan-Kettering Cancer Center announced that it would be excluded from the Center’s treatment protocols because its price was twice as high as the widely used dose of Avastin (bevacizumab) but with no further benefit in overall survival.10 Following delisting by Sloane-Kettering, the manufacturer of Zaltrap halved the price.
In summary, we are beginning to see some resistance to high prices of certain drugs. We can expect more push-back on the part of purchasers, starting in the public sector and spreading to the private sector. Furthermore, orphans are now part of the debate on pricing and whether the cost is justified by the benefit.4,11 A case in point is Kalydeco, which is indicated for cystic fibrosis. The Medicaid agency in Arkansas is implementing step therapy to limit financial exposure.12 Other state Medicaid agencies may follow suit. It should be noted that payers have limited leverage with respect to orphan drug prices because many orphan drugs form unique therapeutic classes where demand is relatively inelastic (ie, not as price sensitive).
As we have seen, high patient cost sharing is a typical payer response, as well as the imposition of more conditions of reimbursement. These are, however, ad hoc reactions to increased spending on orphan drugs. There needs to be a concerted effort to formally evaluate orphan drugs or assess their benefits in relation to cost. Some may have benefits that justify their costs. Others may not. An independent entity, such as the Agency for Healthcare Research and Quality should be given the role of adjudication.
Dr Cohen is research associate professor at the Tufts Center for the Study of Drug Development, Tufts University, Boston, Mass.

References
1. Kaitin KI, ed. Patients face new challenges accessing a growing number of orphan drugs. Tufts Center for the Study of Drug Development Impact Report. 2014;16(4).
2. Orphan Drug Report 2013. EvaluatePharma. Evaluate Ltd., 2013. http://info.evaluategroup.com/rs/evaluatepharmaltd/images/EP_OrphanDrugReport2013.pdf. Accessed August 28, 2014.
3. Silverman E. The new pharmaceutical blockbusters: orphan drugs. Pharmalot. August 23, 2012. http://ycharts.com/analysis/story/the_new_pharmaceutical_blockbusters_orphan_drugs. Accessed August 28, 2014.
4. Cohen JP, Felix A. Are payers treating orphan drugs differently? J Market Access Health Policy. 2014;2:1–5.
5. Cohen JP. Tufts CSDD analysis.
6. Simoens S, Picavet E, Dooms M, et al. Cost-effectiveness assessment of orphan drugs: a scientific and political conundrum. Appl Health Econ Health Policy. 2013;11(1):1–3.
7. McCain J. Could QALYs help in assessing high-priced cancer treatments? Manag Care. 2013;22(12):32–37.
8. Thomson Reuters – Cortellis. The economic power of orphan drugs. Thomson Reuters, 2012. http://thomsonreuters.com/business-unit/science/subsector/pdf/the-economic-power-of-orphan-drugs.pdf. Accessed August 28, 2014.
9. Kelly C. UnitedHealth seeking hepatitis C drug data on when to treat, impact on medical costs. Pink Sheet. 2014;76(31):1–4.
10. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 14, 2012.
11. Hyde R, Dobrovolny D. Orphan drug pricing and payer management in the United States: are we approaching the tipping point? Am Health Drug Benefits. 2010;3(1):15–23.
12. Walker J. Costly vertex drug is denied, and Medicaid patients sue. Wall Street Journal. July 16, 2014.
FDA Sets Date of Advisory Committee Meeting for Donanemab in Alzheimer’s Disease
May 7th 2024The Peripheral and Central Nervous System Drugs Advisory Committee will meet on Monday, June 10, 2024, to discuss the phase 3 trial of Lilly’s donanemab to treat patients with early symptomatic Alzheimer’s disease.
