- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Pharmacologic management of chronic hepatitis B
Chronic hepatitis B is a common disease worldwide with significant morbidity and mortality. Early diagnosis is essential but difficult, as most patients with chronic hepatitis B do not have specific symptoms until the symptoms of advanced disease occur. The main goal of therapy is to prevent cirrhosis and hepatocellular carcinoma by suppressing hepatitis B virus (HBV) DNA levels. The decision to treat and the choice of therapy require careful consideration of both patient and drug characteristics, as stated in both an expert panel consensus recommendation and the American Association for the Study of Liver Diseases (AASLD) treatment guidelines. Lamivudine, once the mainstay of oral antiviral treatment, has been supplanted by newer agents such as adefovir, entecavir, and telbivudine, which produce less drug resistance overall; these oral agents often require long-term treatment. Pegylated interferon alfa-2a, which has a predefined treatment course but is often poorly tolerated, is now the main option in..

Key Points
•Abstract
Chronic hepatitis B is a common disease worldwide with significant morbidity and mortality. Early diagnosis is essential but difficult, as most patients with chronic hepatitis B do not have specific symptoms until the symptoms of advanced disease occur. The main goal of therapy is to prevent cirrhosis and hepatocellular carcinoma by suppressing hepatitis B virus (HBV) DNA levels. The decision to treat and the choice of therapy require careful consideration of both patient and drug characteristics, as stated in both an expert panel consensus recommendation and the American Association for the Study of Liver Diseases (AASLD) treatment guidelines. Lamivudine, once the mainstay of oral antiviral treatment, has been supplanted by newer agents such as adefovir, entecavir, and telbivudine, which produce less drug resistance overall; these oral agents often require long-term treatment. Pegylated interferon alfa-2a, which has a predefined treatment course but is often poorly tolerated, is now the main option in interferon-based therapy, replacing interferon alfa-2b. The goal in the development of future therapies is to improve effectiveness, lengthen the duration of response, and lessen resistance. (Formulary. 2007;42:429–438.)


The natural history of hepatitis B depends on the age of the patient at infection.7 Most children who acquire the virus perinatally do not clear the virus and develop chronic hepatitis B. Most of these children experience an immune-tolerant phase in which the disease is present but clinically silent, resulting in normal alanine aminotransaminase (ALT) levels until adulthood. Immunocompetent adults who acquire acute hepatitis B often clear the virus, except for 3% to 5% who develop chronic hepatitis B.7 The risk of developing chronic hepatitis B is elevated in immunocompromised patients.8
Chronic hepatitis B is often asymptomatic, but nonspecific symptoms of fatigue and anorexia/weight loss may be present. Extrahepatic manifestations are less common and may include arthralgias, purpura, angioedema, and, rarely, vasculitis.9 Patients who develop cirrhosis may develop decompensated liver disease, which may present as jaundice, abdominal distention from ascites, peripheral edema, renal insufficiency, easy bruising and bleeding, confusion, and gastrointestinal varices.
Because most patients with chronic hepatitis B do not have specific symptoms until the symptoms of advanced disease occur, diagnosis is difficult; however, early diagnosis is essential to ensure favorable treatment outcomes. Diagnosis of chronic hepatitis B is made when serum hepatitis B surface antigen (HBsAg) remains positive for >6 months and serum HBV DNA is present.10 Hepatitis B e antigen (HBeAg) is usually positive, but the absence of HBeAg does not preclude the diagnosis, as precore and core promoter mutants suppress expression of HBeAg even as HBV replication occurs. ALT levels are often elevated but may fluctuate over time.
In the setting of chronic hepatitis B, HBeAg may convert from positive to negative (HBeAg clearance), a process that is often accompanied by the development of antibodies to HBeAg (HBeAg seroconversion), reduction in HBV DNA, and normalization of ALT levels.1,11 These events may occur spontaneously or as a result of treatment and usually signal the onset of an inactive HBsAg carrier state. Occasionally, HBeAg is cleared but HBV DNA and ALT levels are intermittently or persistently elevated, which implies the development of precore or core promoter mutants and continued chronic active hepatitis B with HBV replication (HBeAg-negative chronic hepatitis B).
Over time, chronic hepatitis B may lead to progressive liver fibrosis and cirrhosis or hepatocellular carcinoma, with resulting morbidity and increased mortality.12 The HBV DNA levels and the presence of HBeAg are markers for HBV replication and active disease.
PHARMACOLOGIC TREATMENT AGENTS
The goals of anti-HBV therapy are 1) reduction of HBV DNA levels to as low as possible for as long as possible and 2) induction of HBeAg clearance and HBeAg seroconversion to reduce the risk of fibrosis, cirrhosis, liver failure, hepatocellular carcinoma, liver transplantation, and death.12 Suppression or clearance of HBV DNA is now considered the priority in treating hepatitis B, with HBeAg seroconversion as a secondary goal. Other indicators of response include improvement in inflammation on liver histology and normalization of ALT levels.
Older studies of these agents for the treatment of chronic hepatitis B used nonamplified laboratory assays to detect the presence of HBV; the lower limit of HBV detection with these assays was 100,000 copies/mL. Newer, amplified polymerase chain reaction (PCR)-based assays now can detect as few as 50 copies/mL.23 These newer techniques also allow better quantitation of HBV DNA reduction; as a result, trials now often report log10 reduction as a measure of therapeutic response. End points are usually reported at the 1-year mark for the purposes of FDA approval and drug comparison, although the more established drugs have been studied for longer follow-up periods.
Most studies of these approved agents have enrolled patients with chronic hepatitis B who are HBeAg positive, but there is a growing body of literature on patients with HBeAg-negative chronic hepatitis B. These patients have more advanced liver disease and lower rates of spontaneous remission.24 Treatment end points for patients with HBeAg-negative chronic hepatitis B are more difficult to assess, as HBeAg status cannot be monitored. Thus, it is difficult to determine an optimal treatment stopping point for these patients.
Interferon alfa-2b. Interferon alfa-2b was the first FDA-approved treatment for chronic hepatitis B. The glycoprotein has direct antiviral effects and enhances immune response to viruses. The recommended dosage is 5 million units (MU)/d subcutaneously (SC) or intramuscularly (IM) or 10 MU 3 times/wk SC or IM for ≥16 weeks in patients with HBeAg-positive chronic hepatitis B.20
Wong et al25 reviewed the efficacy of interferon alfa-2b in a meta-analysis of 15 randomized, controlled trials involving 837 patients who were administered a 3- to 6-month treatment course of varying doses SC or IM and followed for 6 to 12 months after therapy. At 12 months, HBV DNA was suppressed to <100,000 copies/mL in 37% of patients treated with interferon alfa-2b compared with 17% of those administered placebo. At follow-up, HBeAg clearance had occurred in 33% of patients treated with interferon alfa-2b versus 12% of patients administered placebo, with subsequent HBeAg seroconversion in 18% of patients treated with interferon alfa-2b. Seroconversion was well maintained in 80% to 90% of these patients during long-term follow-up of 4 to 8 years.26,27 During the treatment course or within 6 to 12 months of follow-up, actual elimination of HBsAg, representing a hepatitis B "cure," occurred in 8% of patients treated with interferon alfa-2b and in 2% of patients administered placebo.25 Histologic improvement could not be compared between the 2 groups because of insufficient data.
In 4 randomized, controlled trials enrolling more than 150 patients with HBeAg-negative chronic hepatitis B, treatment with 3 to 5 MU of interferon alfa-2b administered SC or IM 3 times/wk resulted in HBV DNA suppression to <100,000 copies/mL in 40% to 90% of patients at the end of 6 to 12 months of treatment, but this result was counteracted by a high relapse rate, leading to sustained response in only 24% of patients.10 Among those patients who achieved sustained viral response, approximately 30% eliminated HBsAg.
Interferon alfa-2b has been demonstrated to improve clinical outcomes in patients with chronic hepatitis B. Successful treatment with interferon alfa-2b manifests as HBeAg clearance, reduced liver-specific complications, and reduced mortality.28 In a study by Lin et al,29 treatment with 4 to 6 MU/m2 of interferon alfa-2b administered IM reduced the incidence of hepatocellular carcinoma to 1.5% versus 12% in the untreated group.
Unlike treatment with the more recently approved oral agents, treatment with interferon alfa-2b does not result in the development of resistant HBV mutants. However, the frequency and severity of adverse effects, including flu-like symptoms (fever, chills, myalgias, arthralgias, headache, and fatigue), anorexia/weight loss, depression, and bone marrow suppression may result in poor tolerance to this agent.25 As a result, dose reduction is common, and in a study by Wong et al,30 5% of patients required early termination of therapy. Interferon alfa-2b is contraindicated in patients with decompensated liver disease/ cirrhosis, autoimmune diseases, and severe depression or other psychiatric disorders.31 The labeling for interferon alfa-2b contains a boxed warning, which states that interferon alfa-2b may cause or aggravate serious neuropsychiatric, autoimmune, ischemic, and infectious disorders.20 Patients receiving interferon alfa-2b therapy should be monitored periodically with clinical and laboratory evaluations. If patients develop persistently severe or worsening signs and symptoms of these conditions, therapy with interferon alfa-2b should be discontinued.
The ideal candidate for interferon alfa-2b therapy is a patient with elevated ALT levels and low HBV levels who has well-compensated liver disease and sufficient physical and emotional reserve to deal with the possible adverse effects of the therapy. Patients who have been treated with this drug have experienced documented improvement in morbidity and mortality, a significant advantage of treatment.31 Additional considerations may include the desire to avoid drug resistance and a preference for shorter-term therapy. Predictors of interferon alfa-2b response include low serum HBV levels (<200 pg/mL or <600x105 copies/ mL), elevated liver transaminases (ALT >100 IU/L), and HBV genotype A or B (vs C).32,33 Because pegylated interferon alfa-2a, the newer injectable agent, has prolonged therapeutic levels, increased efficacy, and decreased administration frequency compared with interferon alfa-2b, therapy with this newer agent for 48 weeks is likely to replace standard, 16-week interferon alfa-2b therapy in the treatment of chronic hepatitis B.12
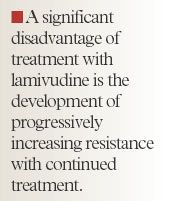
Lamivudine. Lamivudine is a nucleoside analogue that blocks reverse transcriptase and inhibits viral DNA synthesis. The recommended dosage is 100 mg/d orally for ≥1 year, as long-term therapy is necessary to prevent HBV recurrence.21 If HBeAg seroconversion occurs, treatment should be continued for ≥6 months after seroconversion to lower the relapse rate after therapy discontinuation.34
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
