- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
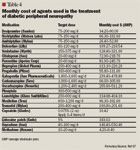
Pharmacologic options for the management of diabetic peripheral neuropathy
Diabetic peripheral neuropathy, one of the most common late complications of diabetes, is associated with decreased quality of life and increased morbidity. The pathophysiology of diabetic neuropathy (DN) is multifactorial, contributing to ischemic and painful events and neuronal damage.
Abstract
Diabetic peripheral neuropathy, one of the most common late complications of diabetes, is associated with decreased quality of life and increased morbidity. The pathophysiology of diabetic neuropathy (DN) is multifactorial, contributing to ischemic and painful events and neuronal damage. Prevention through strict glycemic control remains the mainstay of the treatment of DN, because effective disease-modifying or preventive therapies are not yet available. A vast array of pharmacologic agents such as nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, analgesics, and antiepileptic drugs are used alone or in combination for symptomatic management. These drugs result in moderate effectiveness, potential side effects, and drug interactions.This article will provide a brief overview of the pathophysiology of DN and review pharmacologic agents used for symptomatic management, with a focus on pregabalin (Lyrica, Pfizer) and duloxetine (Cymbalta, Lilly), currently the only agents that are approved by FDA for the pain management of diabetic peripheral neur-opathy. Further research is warranted to assess long-term efficacy of available therapies in the hopes of providing a standard of therapy. (Formulary. 2005;40:438–452.)
Diabetic neuropathy (DN) is recognized as a multifaceted, heterogeneous, neurodegenerative complication of diabetes mellitus (DM), inclusive of type 1 and type 2.1 It is estimated that more than 18 million Americans have diabetes and nearly 50% of them will develop DN over the course of their disease, while roughly 1 in 6 will experience painful DN.2 DN affects numerous tissues and organ systems of the body, thus resulting in significant morbidity and mortality.1
DN is known to affect the sensory, autonomic, and motor neurons of the peripheral nervous system, resulting in major cardiovascular, gastrointestinal, and sensorimotor dysfunction. The true incidence or prevalence is not known, due in part to a lack of agreement on the definition of DN. There are several reasons for this, including variability in clinical manifestations, a lack of consensus regarding clinical criteria to diagnose early neuropathy, and a lack of minimum electrodiagnostic criteria to diagnose subclinical neuropathy.3 The reported prevalence of all forms of DN ranges from as low as 5% to as high as 60% to 100%.4 Patients with diabetes are at risk for developing neuropathies at any given time; however, the incidence increases with duration of the disease, degree of glycemic control, and other contributing factors such as hypertension, hypercholesterolemia, age, body mass index, smoking, and hyperinsulinemia.5–7 Long-term elevation of plasma glucose levels is the primary cause of DN.8,9 It is estimated that the incidence of DN approaches greater than 50% in patients with a history of diabetes of 25 years or longer, thus making it one of the most common diseases affecting the nervous system of patients with a history of long-standing diabetes.10

Sensorimotor neuropathy (SN), also known as distal symmetric polyneuropathy, is the most common type of DN.12 This symmetrical, distal nervous system disorder is linked to degeneration of the distal sensory axons and progresses in a retrograde direction towards the dorsal root ganglia.13 It has an insidious onset and generally affects the upper and lower extremities, causing various degrees of pain or loss of sensation. SN classically presents with progression from the most distal extremities in a symmetrical pattern that is generally described as a "glove-and-stocking" distribution pattern.13,14 Patients often complain of refractory symptoms such as burning, tingling, aching, cold sensation, stabbing sharp pain, numbness, or allodynia.13,14
Peripheral neuropathies are generally progressive and may become irreversible, often resulting in a more severe loss of sensation and increased incidence of foot ulcers, infections, and amputations.15 Most patients with DN describe their symptoms as interfering with sleep, decreasing quality of life, and increasing psychosocial distress.15,16
Two other types of DN include autonomic neuropathy and focal peripheral neuropathy, each affecting various parts of the body in different ways. Autonomic neuropathy may present clinically as loss of sweating in the feet, sexual dysfunction, bladder abnormalities, gastroparesis, orthostatic hypotension, and diabetic diarrhea.17 Focal peripheral neuropathy involves prominent motor involvement associated with a distinct syndrome called diabetic amyotrophy, femoral neuropathy, or sacral plexopathy.14 This syndrome is described as an initial deep jabbing upper leg pain followed by proximal muscle weakness and atrophy.
Only a limited number of patients with DN experience neuropathic pain. Studies of the prevalence of DN give estimates of approximately 34% of diabetic patients, and the prevalence of painful DN may be 11% to 20%.18,19 Many patients do not report symptoms or seek treatment in the course of their disease state until complications are severe and/or perhaps totally irreversible.
Clearly, painful neuropathy can manifest as a serious and costly healthcare burden. Although several treatment modalities are available, it is still difficult to obtain complete pain relief for some patients.20 There remains a need for safe, effective, better-tolerated pharmacologic agents capable of delaying or reversing the progression of nerve damage caused by this devastating disorder. Therefore, the primary focus of this article is to review the pathophysiology of DN and some of the pharmacologic treatments that are used for symptomatic management.
Pregabalin (Lyrica, Pfizer), a gamma-aminobutyric acid (GABA) analog similar to gabapentin (Neurontin, Ivax/Pfizer), is approved for the management of pain associated with diabetic peripheral neuropathy, management of postherpetic neuralgia, and adjunctive therapy for adult patients with partial onset seizures.21 Duloxetine (Cymbalta, Lilly), a reuptake inhibitor of both serotonin (5-HT) and norepinephrine (NE), is approved for the treatment of major depressive disorder and diabetic peripheral neuropathy.22 To date, pregabalin and duloxetine are the only FDA-approved pharmacologic agents for management of diabetic peripheral neuropathy. However, the gold standard of therapy for prevention of DN remains tight glycemic control.8 Although tight glycemic control remains the best means of avoiding complications such as neuropathy, it is evident that not all patients will achieve this goal. There are disease-modifying therapies and preventive agents being studied in phase 2 and 3 clinical trials, but since no such agents are currently on the market, management of neuropathic pain is a key factor of therapy. Effective pain management is important not only to alleviate a patient's suffering; if patients feel better, they may be more physically active, and this can help with glycemic control.
PATHOPHYSIOLOGY
Increasing evidence supports that the pathogenesis of DN is multifactorial. Over the past 20 years there have been 3 main theories to explain DN: the polyol pathway theory, the microvascular theory, and the glycosylation end-product theory.12 It has become increasingly apparent that several pathophysiologic factors probably operate simultaneously, and it may be too simplistic to attempt to explain the many clinical presentations and pathologic findings of DN by a single theory.3
DN is viewed in literature as a microvascular complication of diabetes that arises from alteration in endoneurial and/or epineurial blood vessels leading to absolute or relative ischemia, mainly due to blood vessel cell wall thickening or occlusion7,13,23 This microvascular complication may occur via 1 or more of the following pathways: 1) a metabolic pathway; 2) a neuromuscular insufficiency; 3) an autoimmune damage pathway; or 4) a neurohormonal growth factor deficiency.
The metabolic or polyol pathway results from the hyperglycemic state in which glucose is converted to sorbitol through a series of reactions catalyzed by aldose reductase.24 Excess fructose and sorbitol decrease the expression of the sodium/myoinositol cotransporter, leading to a decrease in myoinosital levels. This causes decreased levels of phosphoinsoitide, which interferes with activation of the Na pump and decreases Na/K ATPase activity. Activation of aldose reductase depletes its cofactor, NADPH, which results in decreased levels of nitric oxide and glutathione, agents that buffer against oxidative injury. Lack of nitric oxide also inhibits vascular relaxation, which can cause chronic ischemia.25
Neuromuscular insufficiency can result from vasculitis, ischemia, or infarction of the individual nerves.26–28 It can manifest as mononeuropathies that are acute in onset and are associated with severe pain, but are usually self-limiting and resolve within 6 to 8 weeks. Nerve entrapment syndromes are compression neuropathies at specific sites in the limb. These sites are narrow anatomic passages where nerves are situated (eg, wrist, elbow). The nerves are particularly prone to extrinsic or intrinsic pressure.29 Carpal tunnel syndrome is the most well-known nerve entrapment syndrome that involves the median nerve and is often described as an occupational disease.
A number of hypotheses have been proposed to explain the targeting of peripheral nerves by diabetes. Loss of sensory neurons may be an irretrievable consequence, limiting options for repair. Alternatively, specific and early approaches that would support the sensory neuron may provide options to prevent such loss and may be accomplished by better understanding the potential role of neuron growth factors.30,31

TREATMENT

Pregabalin. One of the newest of the neuropathic analgesics available is pregabalin. Pregabalin has the same reported mechanism of action as gabapentin (binding to the alpha-2-delta subunit of voltage sensitive calcium), a faster onset of effect, linear pharmacokinetics, and a very high bioavailability.
Duloxetine. Duloxetine was the first pharmacologic agent approved by FDA for treatment of diabetic peripheral neuropathy.33 It demonstrated a higher affinity for human NE and 5HT transporters and a more balanced ratio of NE to 5HT.34 Duloxetine's safety and efficacy for the management of neuropathic pain was established in a multicenter, parallel, double-blind, randomized, 12-week, placebo-controlled, fixed-dose study in adult patients with DN for at least 6 months. 348 patients were randomly assigned to receive either duloxetine 60 mg once daily, duloxetine 60 mg twice daily, or placebo. Both duloxetine-treated groups demonstrated significantly greater improvement (P<.001) compared with the placebo-controlled group after 1 week of therapy using the mean score of 24-hour average pain severity evaluated on an 11-point Likert scale.35

ANTIEPILEPTIC DRUGS
Antiepileptic drugs (AEDs) have been used to control neuropathic pain for more than 50 years.39 Similarities in the pathophysiology and biochemical basis of epilepsy and neuropathic pain have led to their increasing use in the management of neuropathic pain. AEDs are thought to raise the pain threshold and reduce neuronal hyperexcitability involving mechanisms such as blockade of sodium channels, inhibition of glutamate, and enhancement of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA).40
AEDs play a very important role in the treatment of DN; gabapentin and lamotrigine are currently the most frequently prescribed.
Carbamazepine and oxcarbazepine. Carbamazepine (CBZ) (Tegreto/Carbatrol, Taro Pharmaceuticals) a first-generation AED, is often used to control idiopathic trigeminal neuralgia (tic douloureus) and a wide variety of neuropathic pain syndromes.41 Based on clinical evidence, a small number of patients who participated in pivotal trials, and a lack of efficacy when compared with placebo, CBZ is not considered a first-line agent for the treatment of DN.42,43
CBZ's use in clinical practice for pain associated with DN is limited secondarily to its side effect profile.44 Oxcarbazepine (OXC) (Trileptal, Novartis), a newer AED structurally similar to CBZ, has been demonstrated in studies to offer a better side effect profile, not undergo auto-induction, and have fewer drug interactions in its profile.40
Dogra et al45 demonstrated in a randomized, multicenter, double-blind, placebo-controlled trial that OXC was effective in the treatment of painful DN. 146 patients were enrolled in this 18-week trial. Results showed that OXC significantly reduced pain severity from VAS scores (P=.01), more rapid onset of pain relief (P=.02, 22.5 versus 30.7 d), improvement (P=.0025, 48% versus 22%), and lower proportion of nights with awakening because of pain versus placebo (31% vs 49%, P=.02).45
There is a great interest in the newer AEDs (eg, topiramate [Topamax, Ortho-McNeil], levetiracetam [Keppra, UCB Pharma]) as potential therapies for painful DN.44 Early data acquired from experiments in animals and humans suggest that several of these agents have proven efficacy in the treatment of DN.46 However, these agents are being used primarily as therapy for refractory seizures. These newer AEDs offer both improved efficacy in the treatment of refractory seizures, reduction of neuropathic pain and the possibility of reducing or eliminating adverse effects as seen with the older AEDs.46
Like the older AEDs (eg, CBZ), most of the second-generation AEDs possess some ability to block sodium channel conduction as their antiepileptic mechanism of action.46 Tricyclic antidepressants (TCAs), which have proven efficacy in the treatment of neuropathic pain, are also effective sodium channel blockers. It is therefore safe to say that these agents may have proven efficacy in the treatment of neuropathic pain because of an undefined selectivity for certain kinds of sodium channels.47
Gabapentin. Gabapentin was originally developed as a GABA analog, but is believed to act via the blockade of calcium channels. Its effectiveness in DN was shown in 2 large placebo-controlled, randomized clinical trials using doses ranging from 900 mg to 3,600 mg given in divided doses.48,49 Reports indicated that relief of pain was achieved early in therapy at doses >1,800 mg/d.50,51 Morello et al52 demonstrated that when gabapentin (900 to 1,800 mg/d) was compared with amitriptyline (25 to 75 mg/d), there was no difference in analgesic effects. Common side effects included dizziness and somnolence, which were shown to be well-tolerated and similar to placebo. Since gabapentin is excreted unchanged in urine, a dosage adjustment is warranted in patients with renal insufficiency, especially elderly diabetic patients.53
Currently gabapentin is the most frequently prescribed AED for the treatment of neuropathic pain. Some physicians are prescribing a trial of gabapentin before using the older anticonvulsant drugs. Gabapentin, like all other AEDs, is expensive, and its effectiveness is known to diminish over time; for these reasons, many clinicians elect not to use it as first-line therapy.
Lamotrigine. A randomized, placebo-controlled, double-blind, 8-week trial was subsequently conducted in 59 patients with DN.54 Lamotrigine dosing began at 25 mg/d and was titrated gradually to 400 mg/d. Patients recorded pain intensity twice daily using a 0 to 10 scale. Significant reductions in pain intensity scores were realized with lamotrigine (baseline 6.4, treatment 4.2) and placebo (baseline 6.5, treatment 5.3); however, there were statistically significant differences in favor of lamotrigine at dosages of 200 mg/d and higher (P <.001). The reported incidence of adverse reactions in each group was similar.
Lamotrigine has demonstrated some effectiveness in the treatment of neuropathic pain associated with DN in small clinical trails. A large-scale phase 3 trial is currently in progress.
ANTIDEPRESSANTS
Tricyclic antidepressants. Antidepressants have been a traditional first-line therapy for DN. TCAs have been used and studied most extensively for the treatment of DN.42 The issue with TCAs is that they execute several different actions and have multiple receptor activity leading to anticholinergic, antihistaminergic, antiserotonergic, and other effects. The byproducts of amitriptyline (Elavil, Mylan) and imipramine (Tofranil, Aventis) have fewer side effects, so when tricyclics are employed, desipramine (Norpramin, Aventis) and nortriptyline (Pamelor, Watson) are generally used.42
The efficacy of TCAs in managing neuropathic pain is thought to be a result of their analgesic effects rather than antidepressant effects.51 Results from several clinical studies have demonstrated that the relief of neuropathic pain with TCAs does not result from their antidepressant properties. Their analgesic effect is proposed to occur as a result of the reuptake of NE and 5-HT. Anticholinergic adverse effects are often associated with TCAs (eg, dry mouth, constipation, dizziness), while other adverse events may include orthostatic hypotension and sedation.51 TCAs should be used with caution in patients with cardiac disease and the elderly. When choosing a TCA for neuropathic pain therapy, patient tolerability, cost, and risk of adverse drug reactions should be the basis for selection. Literature supports desipramine as the TCA of choice for DN, especially in elderly patients, based on its efficacy, tolerability, adverse effect profile, and cost.55
Selective serotonin reuptake inhibitors. SSRIs have fewer side effects than TCAs, but have been shown to be less effective for the treatment of DN.46 The SSRIs as independent analgesics are controversial. Again, it's difficult to separate out the antidepressant effect from the independent analgesia. Multiple studies show very little, if any, efficacy of SSRIs for the treatment of neuropathic pain when compared with placebo.56,57 With regards to other antidepressants, Semenchuk et al58 demonstrated in a double-blind, crossover study that bupropion-treated patients experienced improvements in neuropathic pain; however, 50% experienced significant adverse effects, such as dry mouth, insomnia, and headache. SSRIs are considered better-tolerated but less effective than TCAs, and should not be considered for monotherapy in the treatment of pain associated with DN.
OPIOID ANALGESICS
Opioids have been used for thousands of years and to this date, remain controversial in neuropathic pain therapy. They produce their analgesic effect through the pre- and post-synaptic interaction with mu-receptors in the CNS.59 Management of neuropathic pain requires large doses of opioids, offering only marginal relief, while causing adverse effects such as sedation, constipation, nausea, hypotension, and development of tolerance and addiction potential. Since painful neuropathy may persist for months, occasionally years, the use of opiates on a continual basis should be avoided. Duby et al1 described opioids as pharmacologic agents providing marginal relief of pain at the risk of severe adverse events; however, studies with sustained release oxycodone (OxyContin, Purdue Pharma) and methadone (Methadose, Roxane) may show potential benefits.
According to a consensus document on the use of opioids, if one has a clear understanding of the terminology as related to opioids, there is a tendency to decrease unnecessary suffering, economic burdens, and adverse consequences for patients and healthcare professionals.60
Oxycodone. Watson et al61 demonstrated in an 8-week crossover study using extended-release oxycodone (Roxicodone, Endo Pharmaceuticals) and placebo in patients with moderate-to-severe pain associated with DN (N=45, P=.01) that oxycodone was more effective than placebo in reducing pain intensity with an average daily dose of 40 mg. Improvement was seen in total pain, skin pain, and disability. The most frequently reported side effects were those regularly seen with opioid use. Any potential benefit of opioids must balance the risk of adverse effects and physical dependence.62 Therapy with extended release opioids requires careful patient selection, regular monitoring of outcomes, and careful dosage adjustment.63
Tramadol. Tramadol (Ultram, Mylan) is an analgesic with a dual mechanism of action: mu opioid agonist and weak inhibition of NE and 5-HT reuptake, leading to the enhancement of endogenous descending pain inhibitory pathways.64 Thus, its mechanism of action is similar to those of narcotic analgesics and antidepressants, which have been effective in treating DN symptoms. Harati et al and Sindrup et al evaluated tramadol for the treatment of DN symptoms.64,65 In each of these randomized trials, participants receiving tramadol reported significantly greater benefits than the placebo groups, including reduced pain and enhanced physical and social functioning. The tramadol groups reported more side effects, most commonly constipation, headache, and drowsiness. Tramadol is not a DEA-scheduled drug and has low abuse and diversion rates.66 It should not be used in patients using other opioids on a chronic basis because of the potential of drug-drug antagonism.

In the second trial, a double-blind, randomized, controlled, crossover trial, Morley et al70 studied the analgesic effectiveness and adverse effect of low-dose methadone in neuropathic pain. The incidence of a daily dose of 10 or 20 mg of oral methadone was evaluated in 18 patients with a diverse range of chronic neuropathic pain syndromes, who had all responded poorly to traditional analgesic regimens. As compared with placebo, the 20-mg daily dose resulted in statistically significant (P=.013–.020) improvements in patients. The VAS ratings of maximum pain intensity, average pain intensity, and pain relief were recorded at the same time daily. The analgesic effects extended over 48 hours, as demonstrated by statistically significant improvements in all 3 outcomes. Analgesic effects were also seen when the lower daily dose of 10 mg of methadone was used, but these failed to reach statistical significance (P=.064 and .065, respectively). Interpatient analysis showed that the analgesic effects were not restricted to any particular type of neuropathic pain. This was the first double-blind randomized controlled trial to demonstrate that methadone has an analgesic effect in neuropathic pain.
NON-OPIOID ANALGESICS
Very little research exists about the effectiveness of over-the-counter analgesics for DN treatment. The lack of evidence is likely based on the fact that patients and practitioners generally report poor efficacy of NSAIDs and acetaminophen when used to alleviate DN. One study compared ibuprofen (600 mg, 4 times/d) and sulindac ([Clinoril, Merck] 200 mg, 2 times/d) and placebo for DN treatment.71 A single-blind design in which each participant (N=18) sequentially used each of the 2 active treatments and the placebo for 8 weeks was used, with participants serving as their own controls. Compared to baseline, sulindac and ibuprofen significantly reduced pain. In contrast, there was no difference in baseline pain and pain reported after 4 or 8 weeks during placebo use. No changes in creatinine or protein levels occurred. The potential for gastrointestinal hemorrhage associated with NSAIDs must be considered, particularly in older adults.30
When NSAIDs are used for pain management in conjunction with other agents, additional pain relief may be obtained, but with the potential of increased side effects, short-term pain relief, and drug interactions. NSAIDs must be used with caution for risk of gastrointestinal and renal complications, along with cardiovascular and cerebral vascular complications as demonstrated by the recent reviews of COX-2 inhibitors.
TOPICAL AGENTS
The use of topical agents to treat painful neuropathy offers several theoretical advantages. There are minimal systemic side effects, no drug-drug interactions, and usually no need for drug titration. The pharmacotherapeutic effect is applied directly to the site of pain generation. Therefore, it is a drug delivery system that can be used very effectively in virtually any patient without having to worry about the risk of drug-drug interactions and toxicity.
Capsaicin. Capsaicin (Capsaicin Cream, Glades), an alkaloid derived from chili peppers, has been used as a topical preparation in strengths of 0.05% and 0.075% cream. Its hypothesized mechanism of action is depleting substance P, a neurotransmitter, and activation of other receptors that may attenuate pain.72 Capsaicin use in treating painful DN was confirmed in a meta-analysis of 4 randomized, controlled trials. Pooled data for 144 participants found capsaicin cream to be 2.75 times as effective as placebo for DN relief (OR, 2.75; 95% CI, 1.73–4.32).73
Topical capsaicin and a TCA were compared in a double-blind, parallel study with 250 participants.74 Statistically significant improvements in pain and activities of daily living were realized in both groups after 8 weeks of therapy (P=.001), and the treatments appeared to be equally effective. Coughing, skin irritation, and rash were side effects seen with patients using capsaicin. This study concluded that topically applied capsaicin is an equally effective but considerably safer alternative to amitriptyline for relief of DN pain. No drug-drug interactions have been reported with capsaicin.75
Lidocaine patch. The lidocaine 5% patch (Lidoderm, Endo Pharmaceuticals) is FDA-approved for postherpetic neuralgia (PHN). Patches are applied for 12 hours on and 12 hours off, with a maximum of 3 patches applied to the affected area in a day. Systemic side effects were reported as minimal, since serum lidocaine levels are not significant. Case reports have also supported lidocaine patches for other types of neuropathic and non-neuropathic pain. However, lidocaine is not considered a preferred medication for the treatment of DN. There is sound evidence to support the quality of life improvements in patients with PHN with the lidocaine patch.76
Barbano et al77 evaluated the effectiveness, tolerability, and impact on quality of life of the lidocaine patch (5%) in an open-label, flexible-dosing, 3-week study with a 5-week extension in an outpatient clinic setting of 56 patients with clinically defined painful DN for longer than 3 months in duration, using a maximum of 4 patches daily for 18 hours. Patients with painful DN showed significant improvements in pain and quality of life outcome measures during the 3-week treatment period. These benefits were maintained in a subgroup of patients treated for an additional 5 weeks, during which tapering of concomitant analgesic therapy was permitted. Adverse events were minimal, and systemic accumulation of lidocaine did not occur. This study concluded that the lidocaine patch (5%) (4 patches daily for 18 h) was well tolerated in patients with painful diabetic polyneuropathy, significantly improved pain and quality of life ratings, and may allow tapering of concomitant analgesic therapy. Given the open-label design of this trial, a randomized, controlled trial is necessary to confirm these results.78
LOCAL ANESTHETICS
Mexiletine. Mexiletine (Mexitil, Teva) is an anesthetic that structurally resembles lidocaine, but unlike lidocaine, it can be given orally. Four studies failed to show a statistically significant improvement in pain, paresthesia, or global assessment compared with placebo.78–81
In the absence of clinical data demonstrating efficacy, mexiletine should only be recommended as an alternative agent for patients suffering from severe, refractory symptoms of peripheral neuropathy minus potential cardiac risk.
FUTURE TREATMENTS
Most of the therapies currently under investigation for DN are based on the pathogenic mechanisms of the disease(Table 5). There are no FDA-approved treatments to modify the natural history of diabetic peripheral neuropathy.

Protein kinase C inhibitors. Ruboxistaurin, a selective protein kinase C (PKC)-beta inhibitor that was in phase 3 clinical trials for the treatment of DN, was discontinued as of August 2005, secondary to demonstrating a lack of efficacy. The ruboxistaurin mechanism of action is thought to be via mediation of several enzymatic signaling systems associated with diabetic neuropathic dysfunction, resulting in decreased progression of DN.84
Erythropoietin. Studies have shown that erythropoietin receptors are found on nerve cells and that perhaps erythropoietin can function as a neuroprotective agent. A recent phase 1/2 clinical trial suggested that erythropoietin can ameliorate neuronal damage in acute ischemic stroke patients.85 Bianchi et al86 demonstrated that intraperitoneal erythropoietin for up to 10 weeks prevented or reversed abnormalities in nerve conduction velocity, NA+ , K+ -ATPase activity, compound-muscle action potentials, nociception, and loss of cutaneous nerve fibers in rats with streptozocin-induced diabetes. However, a potential drawback of erythropoietin therapy in this study was an unwanted increase in red cell mass. Clinicians are aware that any increase in hematocrit could predispose patients to cerebral vascular accidents.
Cannabis sativa-based extract. The Cannabis sativa-based extract Tetranabinex/Nabidiolex (Sativex, GW Pharmaceuticals/Bayer) is a new approach to the treatment of neuropathic pain. This agent contains tetrahydrocannabinol (THC), the main psychoactive ingredient of cannabis, plus cannabidiol (CBD), and an isomer of THC.87
Antioxidants. Clinical evidence supports the use of antioxidants in the pathological and symptomatic treatment of DN.88 Two large multicenter trials to assess the efficacy of alpha-lipoic acid are in progress.
Acetyl-L-carnitine. Acetyl-L-carnitine (ALC) was shown to be deficient in diabetes.89 In preclinical studies, substitution with ALC corrected perturbations of neural Na+/K+-ATPase, myoinositol, nitric oxide (NO), prostaglandins, and lipid peroxidation, all of which play important early pathogenetic roles in DN.
The investigators evaluated frozen databases from two 52-week trials testing 2 doses of ALC, 500 and 1,000 mg (3 times daily), in DN. The 1,257 intent-to-treat patients consisted of 93% of enrolled patients. Primary outcomes were sural nerve morphometry, nerve conduction velocities, vibration perception thresholds, clinical symptom scores, and a VAS for the most bothersome symptom, typically pain. Both studies were evaluated together as well as separately. Compared with placebo, ALC was associated with significant improvements in sural nerve fiber numbers and regenerating nerve fiber clusters. Although vibration perception improved with ALC in both studies, nerve conduction velocities and amplitudes did not improve.

These studies demonstrated that ALC treatment is efficacious in alleviating symptoms, particularly pain, and improved nerve fiber regeneration and vibration perception in patients with established DN. To explore the full effect of ALC on DN, longer trials initiated at an earlier stage of DN are warranted.
Clonidine. A topical form of clonidine is under investigation. Clonidine acts as a local analgesic, and its effect is concentration-dependent.90
Memantine. Memantine (Namenda, Forest), an N-methyl-D-aspartate (NMDA) receptor antagonist, used in patients with moderate-to-severe Alzheimer's disease, is being evaluated for the treatment of neuropathic pain. This drug acts by reducing the neuronal influx of calcium, which contributes to nerve damage. Damaged nerves release excessive amounts of glutamate, which overactivate the NMDA receptors on nerve cell membranes and allow toxic levels of calcium to enter the neurons, causing additional damage.
Neurotropic factors. Neutropic factors in development are generating a great deal of interest. Animal studies have demonstrated their potential efficacy, and clinical trials with nerve growth factor suggest that it somewhat improves neuropathic signs and symptoms, although those findings have not been replicated in large-scale trials.91 While studies of neurotrophic factors continue, research is also focusing on the development of orally administered small molecule drugs that induce the natural expression of nerve growth factors.
DISCUSSION
Estimates by the World Health Organization (WHO) have shown the current global prevalence of diabetes is 3% (194 million people) and is expected to increase in prevalence to 6.3% by 2025.92 It is well-established that the care and treatment of diabetes patients consumes large amounts of healthcare resources.93 In the United States alone, treatment of patients with diabetes is estimated to cost more than $100 billion each year. To enable healthcare payors to budget appropriately, estimating the burden of illness and cost of treatment for diabetes becomes more important as increasing costs stretch limited healthcare resources.93
DN is a common and diverse complication of diabetes that affects the quality of life and life expectancy of patients with diabetes. Pain is one of the most distressing and difficult-to-manage symptoms of DN. However, this may not only be due to a lack of adequate therapies, as demonstrated by Gilron et al.94 Out of 151 patients with neuropathic pain of diverse origin (55.6% diabetic neuropathies) completing a questionnaire, 72.8% complained of inadequate pain control.94 Opioids, TCAs, and AEDs had never been tried by 41.1%, 59.6%, and 72.2% of these patients, respectively, and the newer antineuropathic analgesics were used by only 16.6% of the patients. This study suggested that with new and conventional therapies available, they are often not pursued in patients, despite inadequate pain control.
Antidepressants and anticonvulsants are generally agents with primary indications that do not include pain, but are considered highly effective analgesics. Numerous healthcare providers are becoming more comfortable in using these agents for the treatment of neuropathic pain conditions, including DN. The newer AEDs are being used more now in clinical practice for the treatment of neuropathic pain.
Anticonvulsants have a variety of mechanisms of action that may include activity on the following: voltage-gated ion channels (sodium and calcium channels), ligand-gated ion channels, GABA, glutamate, glycine, combined voltage/ligand channels, and NMDA receptors.95 The primary mechanism of action of AEDs on these various receptors is to reduce ion flow through inhibition of high frequency firing, as found in abnormal Na+ and Ca+ channels. Many of the AEDs are very effective anxiolytics. Gabapentin was shown to be anxiolytic in a number of models, as was pregabalin.95
The SSRIs have demonstrated poor efficacy in most human and animal studies. Rational antidepressant use in neuropathic pain involves using SNRIs or TCAs and avoiding SSRIs.
The historical use, documented effectiveness, and low cost of antidepressants support their use in treating DN. TCAs, primarily amitriptyline and desipramine, have demonstrated significant efficacy in resolving subjective symptoms of this disease over SSRIs; however, due to numerous adverse effects, TCA use is cautioned or is contraindicated in many patients. The novel use of pregabalin and duloxetine, both with limited adverse effect profiles, may supplant the use of other pharmacologic agents as first-line therapies for DN with better treatment and patient outcomes. Comparative long-term and head-to-head studies are still needed to confirm this hypothesis.
With the recent advances in defining the mechanisms involved in neuropathic pain, more effective drug therapy has evolved and will continue to expand towards more effective symptom management and modification and delaying of the neuropathy. While the newer agents, pregabalin and duloxetine, may offer significant benefits in the treatment of DN pain, older agents are still highly utilized by clinicians.
It's important when possible to minimize the number of pharmacologic agents used in treating pain associated with DN in a given patient. If a single agent can treat a condition more effectively, there is the potential for fewer side effects, better compliance, and lower cost.
CONCLUSION
The need for management of pain in patients suffering with DN is important and often requires medical attention. The management of painful diabetic peripheral neuropathy often utilizes an array of pharmacologic agents that can potentiate polypharmacy complications, have moderate effectiveness, and result in frequent treatment failure. Glycemic control remains the only known therapy that reduces the risk and delays the onset of this condition; however, pregabalin and duloxetine have emerged as the only FDA-approved agents for management of pain associated with diabetic peripheral neuropathy. Medications such as recombinant human erythropoietin and ARIs are being investigated for potential disease modification, as well as PKC-beta inhibitors that may contribute to delaying the progression of DN. There is no FDA-approved treatment to modify the natural history of DN. To maximize pharmacologic benefits, it is important to select agents that will treat comorbid diseases, lead to improved function and quality of life, avoid unnecessary polypharmacy, and not lead to addiction. Gabapentin, lamotrigine, tramadol, oxycodone, mexiletine, and acetyl-L-carnitine were the only treatments studied in large, placebo-controlled, parallel-group trials.96 Standardization in design and reporting for comparison of treatments is needed.
Head-to-head trials of current diabetic peripheral neuropathy treatments are lacking, and therefore randomized controlled trials are required to make more informed formulary decisions.
Dr Gauthier-Lewis is an assistant professor at the University of Louisiana at Monroe College of Pharmacy, Monroe, La, in the Department of Clinical and Administrative Sciences, and clinical pharmacist at the LSUHSC-Earl K Long Medical Center, Baton Rouge, La. She can be reached at mlewis7@lsuhsc.edu
. Dr Ibrahim was an assistant professor at the University of Louisiana at Monroe College of Pharmacy at the time the article was written. Dr Riche was a PharmD candidate at the University of Louisiana at Monroe College of Pharmacy at the time the article was written.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Duby JJ, Campbell K. Treatment of painful diabetic neuropathy: a review of the current evidence. US Pharm. 2004;11:10–24.
2. Pirart J. Diabetes mellitus and its complications: a prospective study of 4400 patients observed between 1947 and 1973. Diabetes Metab. 1977;3:97–107.
3. Kelkar P. Diabetic neuropathy. Semin Neurol. 2005;25(2): 168–173.
4. Greene DA, Stevens, MJ, and Feldman, EL. Diabetic neuropathy: scope of the syndrome. Am J Med. 1999;107(2B):2S-8S.
5. Alder AL, Boyko EJ, Ahroni JH, et al. Risk factors for diabetic peripheral sensory neuropathy. Diabetes Care. 1997;20:1162–1167.
6. Boulton AJM, Knight G, Drury J, et al. The prevalence of symptomatic, diabetic neuropathy in an insulin treated population. Diabetes Care. 1985; 8:125–128.
7. Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001; 44:1973–1988.
8. The Diabetes Control and Complications Trial Research Group. The effects of intensive diabetes therapy on the development and progression of neuropathy. Ann Inter Med. 1995; 122:561–568.
9. Thomas PK, Tomlinson DR. Diabetic and hypoglycemic neuropathies. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. Philadelphia, Penn: Saunders; 1993:1219–1250.
10. Greene DA, Sima AAF, Pfeifer MA, Albers JW. Diabetic neuropathy. Annu Rev Med. 1990;41:303–317.
11. Poncelet. AN. Diabetic polyneuropathy: risk factors, patterns of presentations, diagnosis, and treatment. Geriatrics.2003;58:16–30.
12. Feldman EL, Russell J, Sullivan KA, et al. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999; 12:553–563.
13. Baron R. Peripheral neuropathic pain: from mechanisms to syndrome. Clin J Pain. 2000;16(suppl 2):S12–S20.
14. Vinik A, Park TS, Stansberry KB, et al. Diabetic neuropathies. Diabetologia. 2000; 43:957–973.
15. Pinzur MS. The diabetic foot. Compr Ther. 2002; 28:232–237.
16. Eaton, S, Tesfaye S. Clinical manifestaions and measurement of somatic neuropathy. Diabetes Rev. 1997; 7:312–325.
17. Vinik, AL, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care. 2003; 26:1553–1579.
18. Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002; 18:350–354.
19. Simmons Z, Feldman EL. Update on diabetic neuropathy. Curr Opin Neurol. 2002; 15:595–603.
20. Slyke MP. Painful peripheral diabetic neuropathy: therapeutic approaches. Consult Pharm. 2000; 15:544–555.
21. Lyrica [package insert]. New York, NY: Pfizer, Inc; 2005.
22. Cymbalta [package insert]. Indianapolis, Ind: Eli Lilly and Co; 2005.
23. Vinik A. Diabetic neuropathy: pathogenesis and therapy. Am J Med. 1999;107:17S–24S.
24. Thomas PK. Diabetic neuropathy: Mechanisms and future treatment options: J Neurol Neurosurg Psychiatry. 1999; 67:277–281.
25. Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50: 325–329.
26. Kelkar P, Masood M, Parry GJ. Distinctive pathologic findings in proximal diabetic neuropathy (diabetic amyotrophy). Neurology. 2000;55:83–88.
27. Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25:477–491.
28. Said G, Lacroix C, Lozeron P, Ropert A, Plante V, Adams D. Inflammatory vasculopathy in multifocal diabetic neuropathy. Brain. 2003;126: 376–385.
29. Vinik A, Mehrabyan A, Colen L, et al. Focal entrapment neuropathies in diabetes. Diabetes Care. 2004;27:1783–1788.
30. Duby JJ, Campbell K, Setter SM, et al. Diabetic neuropathy: an intensive review. Am J Health-Syst Pharm. 2004; 61;160–176.
31. Toth C, Brussee V, Cheng C, Zochodne, DW. Diabetes mellitus and the sensory neuron. JNeuropathology Experimental Neurology. 2004; 63:561–573.
32. Lesser H, Sharma U, La Moreaux L, Poole RM. Pregabalin relieves symptoms of painful neuropathy. Neurology. 2004;63:2104–2110.
33. Chalon SA, Granier L, Vandenhende FR, et al. Duloxetine increases serotonin and norepinephrine availability in healthy subjects: a double-blind, controlled study. Neuropsychopharmacology. 2003;28:1685–1693.
34. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25: 871–880.
35. Goldstein DJ, Lu Y, Iyengar S, et al. Duloxetine in the treatment of the pain associated with diabetic neuropathy. Presented at: The 156th Annual Meeting of the American Psychiatric Association; San Francisco, Calif; May 17–22, 2003.
36. Wernicke JP, Lu Y, D'Souza DN, et al. Duloxetine at doses of 60 mg QD and 60 mg BID. Presented at: The 56th Annual Meeting of the American Academy of Neurology; San Francisco, Calif; April 24–May 1, 2004.
37. Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psych Res. 2002; 36:383–390.
38. Leo RJ, Barkin RL. Antidepressant use in chronic pain management: is there evidence of a role for duloxetine? Prim Care Companion J Clin Psych. 2003; 5(3):118–123.
39. Woolf CJ, Mannion RJ. Neuropathic pain: etiology, symptoms, mechanism, and management. Lancet. 1999;353:1954–1964.
40. Beydoun A, Kutluay E. Oxcarbazepine. Expert Opin Pharmacother. 2002; 3:59–71.
41. Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbamazepine(Tregretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29:265.
42. Mendell JR, Sahenk Z. Painful sensory neuropathy. N Engl J Med. 2003;348:1243–1255.
43. Rull JA, Quibrera R, Gonzalez-Millan H, et al. Symptomatic treatment of peripheral diabetic neuropathy with carbamazepine (Tegretol): double blind cross over trial. Diabetologia. 1969;5:215–218.
44. Tremont-Lukats IW, Egeff C, Backonja MM. Anticonvulsants for neuropathic pain syndrome. Drugs. 2000;60:1029–1052.
45. Dogra S, et al. Oxcarbazepine for diabetic neuropathy. J Pain. 2004; 5(suppl 1):6.
46. Anderson, GD, Miller, JW. Newer antiepileptic drugs: their collective role and defining characteristics. Formulary. 2001;32:114–135.
47. Backonja M. Use of anticonvulsants for treatment of neuropathic pain. Neurology. 2002; 59(suppl 2):S14–S17.
48. Backonja M, Beydoun A, Edwards ER, et al. Gabapentin for the treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280: 1831–1836.
49. Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trial. Clin Ther. 25:81–104.
50. Rowbothan M, Harde N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842.
51. Collins SL, Moore RA, McQuay HJ, Wiffren P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2002;20:449–458.
52. Morello CM, Leckband SG, Stoner CP. Randomized double blind study comparing the efficacy of gabapentin with amitriptyline in treating neuropathic pain. Arch Intern Med. 1999; 159:1931–1937.
53. Hemstreet B, Lapointe M. Evidence for the use of gabapentin in the treatment of diabetic peripheral neuropathy. Clin Ther. 2001; 23:520–531.
54. Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized controlled study. Neurology. 2001;57:505–509.
55. Max, MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Eng J Med. 1992; 326:1250–1255.
56. Sindrup SH, Gram LF, Brosen K, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain.1990; 42:135–144.
57. Sindrup SH, Bjerre U, Dejgaard A, BrØsen K, Aaes-JØrgensen T, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52:547–552.
58. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 57:1538–1588.
59. Dellemijn P. Are opiates effective in relieving neuropathic pain? Pain. 1999;80:453–462.
60. Savage S, Covington EC, et al. The use of opioids for the treatment of chronic pain: a consensus statement for the American Academy of Pain and American Pain Society. Glenview, Ill: American Pain Society; 2001.
61. Watson CP, et al. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003; 105:71–78.
62. Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology. 2003; 60:927–934.
63. Shah R, Carrig B. Opioids for painful diabetic neuropathy. Am J Health-Syst Pharm. 2004;61: 1446–1447.
64. Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–1846.
65. Sindrup SH, Andersen G, Madsen C, Smith T, Brosen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain.1999; 83:85–90.
66. Harati Y, Gooch C, Swenson M, et al. Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. JDiabetes Complications. 2004;14:65.
67. FDA MedWatch page. Available at: http://www.fda.gov/medwatch/safety/2004/feb_PI/ methadone-PI/ Accessed November 21, 2005.
68. Hays L, Reid C. Use of methadone for the treatment of diabetic neuropathy. Diabetes Care. 2005;28:485–487.
69. Gagnon B, Alrnahrez A, Schreier G. Methadone in the treatment of neuropathic pain. Pain Res Manag. 2003;8:149–154.
70. Morley J, Bridson J, et al. Low dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17:576–587.
71. Cohen KL, Harris S. Efficacy and safety of nonsteroidal antiinflammatory drugs in the therapy of diabetic neuropathy. Arch Intern Med. 1987;147: 1442–1444.
72. Tandan R, Lewis GA, Kruski PB, et al. Topical capsaicin in painful diabetic neuropathy. Diabetes Care. 1992; 15:8–13.
73. Zhang WY, Po ALW. The effectiveness of topically applied capsaicin: a meta-analysis. Eur J Clin Pharmacol. 1994; 46:517–522.
74. Biesbroeck R, Bril V, Hollander P, et al. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Adv Ther. 1995;12:111–120.
75. Halat KM, Denneby CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract.2003;16:47–57.
76. Argoff CE, Galer BS, et al. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Curr Med Res Opin. 2004;20 (suppl 2):S21–S28.
77. Barbano RL, Herrmann DN, et al. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004; 61(6):914–918.
78. Stracke H, Meyer UE, Schumacher HE, et al. Mexelitine in the treatment of diabetic neuropathy. Diabetes Care. 1992; 15:1550–1555.
79. Wright JM, Oki JC, Graves L. Mexilentine in the symptomatic treatment of chronic painful diabetic neuropathy. Ann Pharmacother. 1997; 31:29–33.
80. Dejgard A, Petersen P, Kastrup J. Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet. 1988;2:9–11.
81. Oskarsson P, Lins PE, Ljunggren JG, and the Mexiletine Study Group. Efficacy and safety of mexiletine in the treatment of painful diabetic neuropathy. Diabetes Care.1997; 20:1594–1597.
82. Hotta N et al, and the Diabetic Neuropathy Study Group in Japan. Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. J Diabetes Complications. 1996;10:168–172.
83. El-Kabbani O, Ruiz F, Darmanin C, Chung RP. Aldose reductase structures: implications for mechanism and inhibition. Cell Mol Life Sci. 2004;61:750–762.
84. Way KJ, Katai N, King GL. Protein kinase C and the development of vascular complications. Diabetes Med. 2001;18:945–959.
85. Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505.
86. Bianchi R, Buyukakilli B, Brines M, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA. 2004;101:823–828.
87. Russo, E. Sativex cannabis based medicine maintains improvement in sleep quality in patients with multiple sclerosis and neuropathic pain. Presented at: The American Academy of Neurology 57th Annual Meeting. April 9–16, 2005; Miami Beach, Fla. Presentation p01.061.
88. Barclay L. Alpha-lipoic acid is helpful in diabetic neuropathy. Diabetes Care. 2003; 26:770–776.
89. Sima AA, Calvani M, Mehra M, et al. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic neuropathy: an analysis of two randomized placebo-controlled trails. Diabetes Care. 2005;28:89–94.
90. Max MB, et al. Clonidine in peripheral neuropathy pain relief over time. Comparison of clonidine, codeine, placebo and ibuprofen. Clin Phramacol Therapeutic.1988;43:363–371.
91. Apfel SC, Kessler JA. Neutrotrophic factors in the therapy of peripheral neuropathy. Baillieres Clin Neurol. 1995; 4:593–606.
92. World Health Organization. The World Health Report 2003-Shaping the Future. Available at: http:// http://www.who.int/whr/2003/en/Accessed/ November 20, 2005.
93. Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932.
94. Gilron I, Bailey J, Weaver DF, Houlden RL. Patients' attitudes and prior treatments in neuropathic pain: a pilot study. Pain Res Manag. 2002; 7:199–203.
95. Vinik A. Clinical review: use of antiepileptic drugs in the treatment of chronic painful diabetic neuropathy. J Clin Endocrinol Metab (United States). 2005;90:4936–4945.
96. Adriaensen H, Plaghki L, Mathieu C, Joffroy A, Vissers K. Critical review of oral drug treatments for diabetic neuropathic pain-clinical outcomes based on efficacy and safety data from placebo-controlled and direct comparative studies. Diabetes Metab Res Rev. 2005;21:231–240.
97. First Data Bank. Available at: http:// http://www.firstdatabank.com/. Accessed November 28, 2005.
98. FDC Reports. NDA Pipeline. Available at: http:// http://www.ndapipeline.com/. Accessed November 20, 2005.
