- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Pramipexole: A nonergot-derived dopamine agonist for the treatment of restless legs syndrome
Drugs that act to increase dopamine activity are the mainstay of pharmacologic treatment for restless legs syndrome (RLS), a sensomotor disorder that usually manifests as an urge to move the legs, with or without other uncomfortable sensations.
Abstract
Drugs that act to increase dopamine activity are the mainstay of pharmacologic treatment for restless legs syndrome (RLS), a sensomotor disorder that usually manifests as an urge to move the legs, with or without other uncomfortable sensations. Pramipexole, a nonergot-derived dopamine agonist previously approved by FDA for the treatment of Parkinson disease, received FDA approval for the treatment of moderate-to-severe RLS in November 2006. In placebo-controlled clinical trials, treatment with pramipexole improved RLS symptom scores and measures of sleep quality. Common adverse events included nausea, fatigue, headache, and somnolence. Sudden onset of sleepiness has also been reported rarely in patients being treated with pramipexole for RLS. Augmentation, a change in or worsening of symptoms after initiation of treatment, may occur in up to one-third of patients treated with this agent. More data are needed to better characterize the relative efficacy of pramipexole compared with other treatments for RLS and to clarify the rates of less common adverse events. (Formulary. 2007;42:165–174.)

In population surveys, approximately 10% of people report the presence of RLS symptoms on ≥5 nights each month, although some estimates place the prevalence of RLS in primary care practices closer to 15%.2,3 RLS appears to be more common in patients of Northern or Western European descent than in patients of Asian descent.3
RLS may be primary (idiopathic) or may develop secondary to other conditions such as chronic renal failure, iron deficiency, or pregnancy. Of these conditions, iron deficiency is most commonly associated with RLS, but it is unclear if RLS is only associated with iron deficiency or if low iron stores somehow lead to dysfunction in the dopamine system.3 One algorithm for the management of RLS published in 2004 recommended assessment of serum ferritin concentration, with ferrous sulfate replacement therapy recommended for patients with low-to-normal levels.2 Hypothyroidism, diabetes mellitus, rheumatoid arthritis, and Sjögren's syndrome have also been associated with RLS symptoms, although these associations are not well established.2–4 Symptoms of secondary RLS often remit if the contributing condition is resolved, as in the case of renal transplant or the end of a pregnancy.1,5
Current thinking has focused attention on the role of the dopaminergic system in the development of RLS symptoms. Some, though not all, studies have found modest reductions in brain dopamine function in patients with RLS.2,3 Also, use of medications that affect brain dopamine levels, including antidepressants, neuroleptic agents, and antiemetics, may contribute to RLS symptoms.2,3 It has been proposed that dopamine-related changes in behavior and mood are mediated by the D3 dopamine receptor, whereas motor effects are the result of D2 receptor activity.6 Increased dopaminergic activity as a result of treatment with levodopa or dopamine agonists has been demonstrated to suppress RLS symptoms, and symptom response to dopaminergic therapy supports the clinical diagnosis of RLS.1–3 As a result of this association between RLS symptoms and dopaminergic therapy, the nonergot-derived dopamine agonist pramipexole (Mirapex, Boehringer Ingelheim), which was previously approved by FDA for the treatment of Parkinson disease, received FDA approval in November 2006 for the additional indication of treatment for moderate-to-severe RLS, joining ropinirole (also a nonergot-derived dopamine agonist, approved for RLS in 2005) as a new treatment option for RLS.7–9
Despite the approval of these therapies specifically for RLS, the natural history of RLS is not well known, as most patients with mild or intermittent symptoms do not seek treatment. Symptoms may also vary greatly from patient to patient. In younger patients (aged <50 years), symptoms are typically insidious in onset, whereas symptom onset is usually acute in older patients. Regardless of the time to symptom onset, RLS is usually a chronic problem and may significantly affect the patient's sleep by causing difficulties in falling asleep (increasing sleep latency), reducing sleep time, or waking the patient from sleep.1
There is no standard measure to monitor RLS. Published clinical trials have assessed symptoms using several criteria. The Clinical Global Impression (CGI) and Patient Global Impression (PGI) scales rate symptom severity by assigning 1 of 7 ratings to symptoms, from "very much improved" to "very much worse." The 10-item International RLS Study Group (IRLS) scale is a validated instrument that patients use to self-classify RLS symptoms. Disease severity is classified as mild (score range, 1–10), moderate (range, 11–20), severe (range, 21–30), or very severe (range, 31–40). Published trials have also used several measures of quality of life (QOL), including the Restless Legs Syndrome Quality of Life (RLS-QOL) questionnaire (range, 0 [worst]–100 [best]) and general QOL measures such as the Short Form-36 (SF-36). Researchers often evaluate the effect of RLS on patients' sleep by analyzing sleep diaries or using standardized scales. Polysomnography (performed in a sleep laboratory) is also used to assess various sleep parameters and to record PLMS and PLMS index (the number of PLMS per hour).3,5
Once RLS has been diagnosed, healthcare professionals should recommend nonpharmacologic strategies and should assess possible secondary causes of RLS before initiating drug treatment for RLS. When possible, aggravating medications should be discontinued or doses should be reduced. Patients should be encouraged to practice good sleep habits; to avoid caffeine, nicotine, and alcohol; and to try engaging in activities such as puzzles or videos that increase mental alertness during times of boredom.2,4
Not every patient requires drug therapy, and treatment should be tailored based on patient complaints, symptom severity, and comorbid conditions. For bothersome RLS symptoms that persist despite trials of nonpharmacologic strategies, agents that increase dopamine activity, including carbidopa/levodopa and the dopamine receptor agonists ropinirole and pramipexole, have been demonstrated to reduce symptoms. A 2004 Academy of Sleep Medicine report described practice parameters (including off-label use of medications) for the use of dopaminergic agents to treat RLS.10 These guidelines used standard methods to grade available evidence for RLS treatments and to present the recommended treatments as either standard (treatment supported by a high degree of clinical certainty), guideline (a patient care strategy supported by a moderate degree of clinical certainty), or optional (treatment with uncertain clinical use). The practice parameters classified levodopa/carbidopa, pergolide, pramipexole, and ropinirole as effective for the treatment of RLS. At the time of publication of the guidelines, recommendations for levodopa/ carbidopa and pergolide were offered at the standard level, treatment with pramipexole at the guideline level, and treatment with ropinirole at the optional level. The algorithm for the management of RLS published in 2004 (which also included off-label use of medications) recommended the use of levodopa/carbidopa for the treatment of intermittent RLS symptoms and the use of dopamine receptor agonists such as pramipexole and ropinirole for the treatment of daily RLS.2

Since publication of the 2004 practice parameters, both ropinirole and pramipexole have gained on-label FDA approval for the treatment of RLS.
CHEMISTRY AND PHARMACOLOGY
Pramipexole is a nonergot-derived agonist at dopamine D2 receptors with strong affinity for the D3 receptor subtype; this agent has greater affinity for the D3 receptor subtype than ropinirole, but it is unclear how that relates to the clinical use of these agents.6 Pramipexole has very little activity at D1 receptors or at serotonin (5-HT2A or 5-HT2B) receptors.6,7 A more specific mechanism for the action of pramipexole in RLS has not been well characterized.
PHARMACOKINETICS
Pramipexole has an absolute bioavailability >90% and achieves peak concentrations in 2 hours. Administration of pramipexole with food delays the time of maximum concentration but does not affect the extent of absorption. Pramipexole does not appear to undergo significant first-pass metabolism.7 It has a high volume of distribution and is approximately 15% protein bound. Pramipexole is largely excreted as unchanged drug and is secreted by the renal tubules with clearance of approximately 400 mL/min. Renal impairment may have a significant effect on the pharmacokinetics of pramipexole. The half-life of pramipexole is approximately 8 hours in healthy volunteers and 12 hours in elderly patients.7
CLINICAL TRIALS

Most recently, Montplaisir et al18 published the results of a telephone survey that evaluated the long-term efficacy and adverse events of pramipexole therapy in patients with no previous exposure to dopaminergic agents. Clinic records were reviewed to identify eligible patients who had a diagnosis of primary RLS and were undergoing pramipexole treatment that had been initiated >12 months earlier. A total of 196 patients were eligible for the study, and 195 patients agreed to participate. At the time of the telephone survey, 152 patients were still taking pramipexole; the remainder had discontinued treatment because of adverse events (n=20), lack of efficacy (n=6), both (n=6), or other reasons (n=11). Patients who had discontinued were more likely to have been diagnosed with milder disease before treatment than patients who were still taking pramipexole. The mean duration of pramipexole therapy for patients who were still taking the drug at the time of the survey was 30.5±10.5 months. Of those who had continued treatment, the mean pramipexole dose was 0.59±0.31 mg (range, 0.125–2.25 mg), and the mean RLS symptom severity score had decreased from baseline by 80%±20.8%. Ninety-four percent of patients reported a decrease in symptom severity of ≥50%. Patients also reported improvements in RLS symptoms that had caused them to wake at night or to have difficulties in falling asleep. The authors concluded that pramipexole was effective for the long-term treatment of RLS in drug-naïve patients.
Pramipexole was also evaluated in an open-label study in patients with RLS secondary to renal insufficiency.19 Ten patients on hemodialysis who had RLS symptoms severe enough to interfere with dialysis sessions were treated with pramipexole (dose range, 0.125–0.5 mg) and were followed for a mean of 8 months. Primary efficacy measures included changes in the IRLS score and PLMS index. Mean IRLS scores fell from a baseline of 25.8±5.75 to 7.7±8.36 (P<.005) after treatment, and the mean PLMS index was reduced from 108.7±42.38 at baseline to 38.4±27.46 after treatment (P<.001). No significant differences were found on other measures of sleep quality. The authors suggested that pramipexole was effective for the treatment of secondary RLS in patients undergoing hemodialysis, but this indication requires additional study.
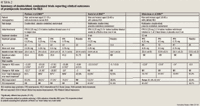
The first randomized, controlled trial to evaluate pramipexole for the treatment of RLS was published by Montplaisir et al in 1999.20 This 10-week, double-blind, placebo-controlled, crossover study enrolled 10 men and women aged 20 to 65 years with RLS as defined by the RLS Study Group. Patients were excluded if they had any condition associated with RLS, had a PLMS index <10, had any other sleep disorders, or were using any other medications that might affect sleep patterns in the 2 weeks before the study. The study design included two 4-week treatment periods with a 2-week washout between periods. Patients spent four 2-night evaluation periods in a sleep laboratory at baseline and at the end of Weeks 4 (first treatment period), 6 (washout), and 10 (second treatment period). The primary outcomes of the study were the PLMS and PLMW indices from the sleep laboratory recordings. Secondary outcomes included sleep latency and efficiency. Patients received pramipexole or placebo tablets 1 hour before bedtime. The initial dose of pramipexole was 0.375 mg, with increases after 1 and 2 weeks to a final dose of 1.5 mg.

The authors studied 7 of the patients who completed this study in a separate trial to determine if the therapeutic effect of pramipexole continued after 6 months of continuous treatment and if patients treated with pramipexole developed rebound morning restlessness or augmentation.26 Pramipexole therapy was initiated at 0.25 mg 1 hour before bedtime, and the dose was increased weekly until symptom relief was obtained. The mean effective dose was 0.5 mg. Patients completed questionnaires to record the presence of symptoms at various times throughout the day. The authors observed a significant reduction (P=.028) in leg restlessness at bedtime and nighttime after 1 month of treatment with pramipexole. There was no evidence of a decrease in effect after long-term use (mean, 7.8 months) and no evidence of morning rebound or augmentation.
Saletu et al21 performed a single-blind, placebo-controlled, unbalanced, crossover sleep laboratory study to determine the acute effects of pramipexole when used to treat RLS. Eligible patients were those who had a diagnosis of RLS with stable symptoms for the 2 weeks before the study. Patients were excluded if they had another sleep disorder, neuropathy, or various psychiatric diagnoses that might affect RLS symptoms. Patients were evaluated on 3 nights: a pretreatment night, a placebo night, and a drug night. Patients received 0.088 mg pramipexole base (equivalent to 1.25 mg dihydrochloride) at 9:00 PM and 0.18 mg pramipexole at 10:30 PM on the drug night. Various sleep quality measures were recorded overnight, including PLMS, total sleep time, sleep efficiency, sleep stages, and sleep latency to each stage. The primary outcome was the measure of PLMS per hour of total sleep time. Patients also completed subjective assessments of sleep and RLS symptoms and mental performance tests upon awakening. Patients then entered a 4-week, open-label, follow-up phase in which the dose of pramipexole was titrated to optimal effect or until further increases were limited by persistent adverse events. Efficacy was assessed with the IRLS scale.
Eleven patients (8 men and 3 women) were enrolled in the study, and all but 1 completed the 4-week follow-up period. In the acute phase, the primary outcome of PLMS index was improved from 35.0±55.5 at baseline to 8.2±6.1. Pramipexole also improved total sleep time by a median of 18% (P=.017) and improved sleep efficiency by a median of 19% (P=.002) compared with placebo. The investigators concluded that, overall, pramipexole treatment resulted in significant differences (P<.05) in objective measures of sleep quality compared with placebo. Patients' subjective reporting of sleep quality was also improved with pramipexole treatment. There were no differences between pramipexole and placebo on mental performance the morning after treatment. After 4 weeks of follow-up treatment, IRLS scale scores improved from a pretreatment score of 23±7 to 13±10 (P=.004).
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
