- Safety & Recalls
- Regulatory Updates
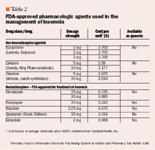
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Ramelteon: A novel melatonin receptor agonist for the treatment of insomnia
Ramelteon (Takeda Pharmaceuticals) is a selective melatonin receptor agonist awaiting FDA approval for the treatment of insomnia. Unlike the currently prescribed hypnotic agents that work by targeting gamma-aminobutyric acid (GABA) receptors, ramelteon offers a novel mechanism of action, specifically targeting the MT1 and MT2 receptors in the brain, which are thought to play a role in regulating sedation and circadian rhythms. Ramelteon is characterized by undergoing extensive first-pass metabolism along with having high oral bioavailability. Results of phase 2 clinical trials have demonstrated that ramelteon-treated patients had significantly shorter sleep onset latencies and longer total sleep times than placebo-treated patients. Furthermore, ramelteon therapy did not appear to impair patient cognition, memory recall, levels of alertness, or ability to concentrate. Data from animal studies suggest that ramelteon is not likely to cause abuse or physical dependence. Unlike the current FDA-approved..
Abstract Ramelteon (Takeda Pharmaceuticals) is a selective melatonin receptor agonist awaiting FDA approval for the treatment of insomnia. Unlike the currently prescribed hypnotic agents that work by targeting gamma-aminobutyric acid (GABA) receptors, ramelteon offers a novel mechanism of action, specifically targeting the MT1 and MT2 receptors in the brain, which are thought to play a role in regulating sedation and circadian rhythms. Ramelteon is characterized by undergoing extensive first-pass metabolism along with having high oral bioavailability. Results of phase 2 clinical trials have demonstrated that ramelteon-treated patients had significantly shorter sleep onset latencies and longer total sleep times than placebo-treated patients. Furthermore, ramelteon therapy did not appear to impair patient cognition, memory recall, levels of alertness, or ability to concentrate. Data from animal studies suggest that ramelteon is not likely to cause abuse or physical dependence. Unlike the current FDA-approved hypnosedatives, ramelteon has been evaluated in trials for periods of up to 1 year. Results from multiple phase 1/2 studies indicate that the most commonly reported adverse effects include somnolence, headache, fatigue, nausea, and dizziness. (Formulary. 2005;40:146-155.)
Insomnia is a prevalent disorder of unsatisfactory or poor quality sleep and is associated with adverse daytime outcomes.1 It presents as difficulty falling asleep (sleep latency), difficulty maintaining sleep (frequent awakenings), or as nonrestorative sleep. Insomnia has been attributed to numerous factors, including stress, acute and chronic medical conditions, psychiatric illness, changes in setting, or certain medications.2 Insomnia is classified according to the duration of symptoms, with transient and short-term insomnia representing the most common manifestations of sleep disturbances. By definition, transient is categorized as occurring a few nights and short-term refers to a duration of 3 to 4 weeks. Insomnia lasting longer than 4 weeks is considered chronic and is usually the result of a medical or psychiatric condition.1,2
Management of insomnia depends on the type of insomnia and frequency of symptoms. Primary interventions, including sleep hygiene training, relaxation training, and stimulus-control therapy, should be recommended first to patients.2 Sleep hygiene helps establish proper daily habits to promote sleep and minimize habits that interfere with sleep such as avoiding caffeine consumption and eating or exercising immediately before bedtime. The use of relaxation techniques such as progressive muscle relaxation, meditation, or yoga may reduce psychic and muscular tension that will interfere with sleep onset. Stimulus-control therapy is another behavioral approach that helps reduce arousing stimuli in the bedroom, thus improving sleep efficiency.2
If primary interventions are ineffective or partially effective in controlling insomnia, pharmacologic agents and cognitive behavior therapy (CBT) can be used.2 Drugs vary in onset of action, half-life, and side effect profile, so the selection of an agent will be dependent on the patients' presentation of insomnia and comorbid conditions. Zolpidem (Ambien, Sanofi-Aventis) and zaleplon (Sonata, King Pharmaceuticals) are agonists at the benzodiazepine receptor component of the gamma-aminobutyric acid (GABA) receptor complex. They are rapidly absorbed and well-tolerated with low risk for tolerance and abuse potential. Sleep latency of zaleplon is about 10 to 20 minutes with a duration of action of of approximately 4 hours; therefore, zaleplon is effective in restoring sleep in patients with nocturnal awakenings.5 Zolpidem has an onset of action within 30 minutes and its hypnosedating effects last up to 8 hours.6
Other commonly prescribed hypnotics are benzodiazepines such as flurazepam and temazepam.2 Benzodiazepines are safe and effective for the short-term treatment of insomnia; however, risks of morning sedation, memory dysfunction, development of tolerance, and misuse limit long-term treatment. Antidepressants may be used for insomnia, including tricyclic antidepressants such as amitriptyline, doxepin, and trazodone; however, antidepressants are not approved by FDA for use in the treatment of insomnia, as there is a lack of controlled clinical trial data validating the efficacy of these agents.2 Over-the-counter medications containing antihistamines such as diphenhydramine are commonly used, but their efficacy is not established for the treatment of insomnia. Exogenous melatonin has also been used for insomnia, as the endogenous hormone is found to be involved in the control of circadian rhythm. Unfortunately, several studies examining the effectiveness of melatonin yielded mixed results.2 Nonpharmacologic therapy such as CBT can be used alone or in conjunction with drug therapy for optimal results. CBT, which requires specially trained clinicians, has been shown to be effective for the treatment of chronic insomnia. CBT focuses on altering dysfunctional beliefs and thoughts about sleep that cause insomnia.2
The advancement of the treatment of insomnia has led to the development of several new agents. Eszopiclone (Lunesta, Sepracor) is a new non-narcotic agent recently approved by FDA for the treatment of insomnia. Eszopiclone has been shown in a 6-month trial to improve sleep latency, wake time after sleep onset, number of awakenings, total sleep time, and quality sleep. Eszopiclone is well-tolerated and has not been shown to develop tolerance.7 Gaboxadol8 and indiplon9 belong to a class of GABAA agonists that is currently being investigated for the treatment of sleep disorders.
Ramelteon (Takeda Pharmaceuticals), a selective MT1 (melatonin) receptor agonist, is a novel hypnotic agent under FDA review. In late September 2004, a new drug application (NDA) was submitted to FDA for ramelteon in the treatment of insomnia.10 A thorough evaluation of the pharmacology, clinical trial results, safety, and tolerability of ramelteon is presented in this review.
CHEMISTRY AND PHARMACOLOGY Ramelteon ((S)-N-[2-(1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-yl)ethyl]propionamide) is a tricyclic indan derivative with a molecular weight of 259.34 d. This chemical entity has been shown to have a high affinity for certain human melatonin receptors.11
Three subtypes of mammalian melatonin receptors have been identified and are designated as MT1, MT2, and MT3. MT1 and MT2 are known to be involved in the regulation of sleep and have distinct roles in the suprachiasmatic nucleus (SCN). The SCN is located in the hypothalamus and functions as the "master clock," regulating the 24-hour sleep-wake cycle through the day and night phases. MT1 is thought to regulate sleepiness, while MT2 is more likely to be involved in the readjustment of circadian rhythms.12,13 Melatonin receptors belong to the G-protein-coupled receptor family, characterized by having 7 membrane-spanning domains.12 The activation of these receptors by melatonin is coupled to the inhibition of adenylate cyclase and a consequent decrease in intracellular cyclic adenosine monophosphate (cAMP) levels.11 Hence, a cAMP-dependent signal transduction cascade may account for ramelteon-induced pharmacologic effects.13
Ramelteon is a selective melatonin receptor agonist with a high affinity for MT1 and MT2 receptors and a low affinity for MT3 receptors. Results from animal studies using chick forebrain MT1 receptors and hamster MT2 receptors showed that the selectivity of ramelteon for MT1 over MT2 was more than 1,000-fold greater than that of melatonin.14 This suggests that ramelteon may act more specifically on the sleep-onset process than melatonin. In addition to exhibiting selective affinity for MT1 receptors, ramelteon has been shown to have a 3- to 6-fold higher affinity for human MT1 and MT2 receptors (expressed in Chinese hamster ovary cells) in comparison to melatonin, with inhibitory rate constants (Ki) of 14.0 pM and 112 pM, respectively.13
The abuse potential and the effects of ramelteon on cognitive function have been evaluated in a recent study conducted by Miyamoto et al.15 The researchers examined the binding affinities of ramelteon and its active metabolite (M-II) to other potential binding sites such as receptors for neurotransmitters, peptides, and cytokines, as well as ion channels and various enzymes. The results showed that ramelteon had no measurable affinity for any large number of ligand binding sites, including benzodiazepine, dopamine, and opiate receptors. Consequently, the investigators concluded that ramelteon exerts sleep-promoting action without causing learning and memory impairment, motor dysfunction, and drug-abuse ability.
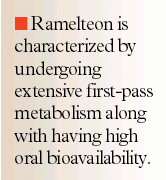
A dose-ascending study conducted in 60 healthy subjects (aged 35-65 y) reported that the pharmacokinetics of ramelteon were dose-proportional.19 Subjects were randomly assigned to receive single oral doses of ramelteon (4, 8, 16, 32, and 64 mg) or placebo. Blood samples drawn during the 16-hour interval post-dose were used to measure AUC, Cmax, t1/2, and Tmax. The results revealed that mean AUC values increased with dose escalation (ranging from 1.7 hr-ng/mL with the 4-mg dose to 36.1 hr-ng/mL with the 64-mg dose). A similar trend was seen with Cmax values (ranging from 1.1 ng/mL to 25.9 ng/mL, respectively) and the mean elimination t1/2 for ramelteon. While t1/2 was dose-dependent, it remained relatively short, ranging from 0.83 to 1.9 hours. In contrast, mean Tmax appeared to remain comparatively constant with increasing doses (values ranged from 0.78 to 0.94 h).
The effect of food on ramelteon pharmacokinetics was studied in 24 healthy subjects randomized to receive oral ramelteon 16 mg with and without food.20 Results from the pharmacokinetic samples collected over the 24-hour period following ramelteon administration demonstrated that the presence of food increased AUC by 31% (90% CI, 109%-157%) but decreased Cmax by 22% (90% CI, 57.8%- 105%) and also delayed Tmax by 55 minutes (P<.001). Mean t1/2 did not appear to be affected by food. Despite the changes found in AUC, Cmax, and Tmax when ramelteon was given with food, the changes are not considered clinically significant because of ramelteon's wide therapeutic window. Therefore, ramelteon may be administered with or without food.
In another phase 1 study (N=48), Greenblatt et al evaluated the effects of age and gender on ramelteon's pharmacokinetics.21 Subjects were divided into 4 study groups: young (aged 18-34 y), male and female, and elderly (aged 63-79 y), male and female. Following a 16-mg single oral dose, plasma concentrations of ramelteon and its metabolite, M-II, were analyzed from blood samples obtained during the 24 hours following administration. Study results indicated that regardless of gender, AUC values for both ramelteon and M-II in elderly subjects were significantly elevated compared with young subjects and clearance was significantly decreased for the same group comparison. Additional data presented at a later date revealed that not only was AUC increased in elderly subjects, but Cmax and t1/2 values of both ramelteon and M-II were also significantly elevated (AUC, 18.7+19.4 vs 10.5+12.8 ng-h/mL, P=.001; Cmax, 11.6+13.8 vs 6.9+7.6 ng/mL, P=.024; t1/2, 2.6+1.14 vs 1.57+0.778 h, P=.004, for ramelteon and M-II, respectively).22
Single- and multiple-dose ramelteon pharmacokinetics were also assessed in subjects with hepatic or renal impairment. In the hepatic impairment study, 48 subjects were divided based on hepatic function (as determined by Child-Pugh [CP] score) and received ramelteon 16 mg orally (single dose cohort) followed by a 2-day washout period and then ramelteon 16 mg daily for 5 days (multiple dose cohort).23 Following both single and multiple dose ramelteon, AUC and Cmax were found to be increased (5- to 11-fold) in subjects with mild (CP 5-6) and moderate (CP 7-9) hepatic dysfunction compared with matched healthy controls. However, the degree of hepatic impairment did not correlate well with the degree of increase in ramelteon exposure. Despite the significant increase in ramelteon exposure in subjects with hepatic dysfunction, the authors noted that the levels still remained within ramelteon's inter-individual variation and therefore no dosage adjustment is recommended for these patients. Tolbert et al also conducted a renal impairment study that enrolled 50 subjects (renal impairment grouped by creatinine clearance: mild, 50-80 mL/min; moderate, 30-49 mL/min; severe, <30 mL/min; and hemodialysis) and followed a similar ramelteon dosing schedule as that done in the hepatic impairment study.24 Data revealed that neither single nor multiple dose ramelteon were significantly affected by renal dysfunction, except in the severely impaired group, which showed a 2- to 4-fold increase in ramelteon exposure. Based on the pharmacokinetic data of this study, the authors concluded that ramelteon would not require dose adjustment in renally impaired patients.
CLINICAL TRIALS Ramelton (formerly TAK-375) has been in development since 1997 for the management of insomnia in a variety of settings. The manufacturer currently has results from 43 ramelteon trials in the company database.
Ramelteon's effects on sleep were studied in both freely moving cat and monkey models. In the cat model, ramelteon (0.001, 0.01, and 0.1 mg/kg po) was shown to promote sleep when administered during the daytime.11 Ramelteon's sleep-inducing effects did not appear to affect sleep architecture as opposed to benzodiazepines and the newer benzodiazepine-receptor agonists, which have been shown to decrease rapid eye movement (REM) sleep. A study of ramelteon (0.03 and 0.3 mg/kg po) in monkeys produced similar sleep-promoting effects on nocturnal sleep.25
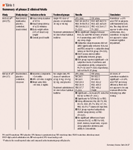
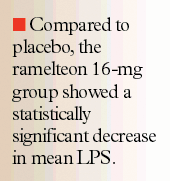
No significant differences in demographics and baseline characteristics were noted among the treatment groups. Compared to placebo, the ramelteon 16-mg group showed a statistically significant decrease in mean LPS (24.6+21.9 vs 14.1+ 15.1 min, respectively, P<.001). Similarly, the ramelteon 64-mg group demonstrated a significant decrease in mean LPS (P<.001 compared to placebo). Both ramelteon dosage groups were associated with increased TST (P=.007 and P=.033 for the 16- and 64-mg ramelteon groups, respectively), but were not significantly different from placebo with regards to percentage of TST spent in each sleep stage, WASO, and number of awakenings. Subjects in the ramelteon 16-mg group reported shorter mean subjective sleep latency compared to those in the placebo group (P=.013); however, no significant difference was found between the ramelteon 64-mg group and placebo.
In the second study, Erman et al evaluated the efficacy of ramelteon (4, 8, 16, and 32 mg), given as 2 daily doses, for primary chronic insomnia as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).27 The primary efficacy end point was the objective difference in LPS as measured by PSG. Similar to the results reported in the Roth et al study, a statistically significant reduction in LPS was found at all doses of ramelteon. Mean LPS values for ramelteon 4, 8, 16, and 32 mg were 24, 24.3, 24, and 22.9 minutes, respectively, which were significantly lower compared with placebo (mean LPS, 37.7 minutes, P<.001). Additional data revealed that all dose groups of ramelteon significantly increased TST and sleep efficiency (P<.001 for both comparisons vs placebo). No significant differences were found between all treatment groups in the assessment of awake time after sleep onset, subjective TST, sleep quality, and cognitive function. Of note, reported subjective sleep latency in the ramelteon 16-mg dosage group was significantly shorter compared with placebo (P=.015).
Phase 3. Although an NDA for ramelteon was submitted to FDA in September 2004, phase 3 clinical trial data have yet to be published. Effects of single daily doses of ramelteon administered for various time periods up to 1 year were studied in more than 4,200 patients aged 18 to 93 years.10 Trials were conducted in the United States, Japan, and Europe, and included 7 placebo-controlled trials. In addition, the company has interim safety data from an ongoing, year-long study.
Conflicting data were reported for the comparison of zolpidem and temazepam in regard to sleep outcomes. In one study evaluated by the meta-analysis, no significant differences between the 2 treatments were noted for sleep latency, while another study reported a statistically significant decrease in sleep latency with zolpidem as well as improvement in subjective quality of sleep. 28 There also did not appear to be any significant differences in adverse events and effects on daytime alertness between the 2 treatments. In comparison, zopiclone did not appear to be significantly different from temazepam in terms of effects on sleep latency, duration of sleep, number of awakenings, quality of sleep, adverse effects, and daytime alertness. One study noted no significant difference between zopiclone and temazepam with regard to rebound insomnia, although a second study indicated that zopiclone had significantly more rebound insomnia of sleep onset latency.
Only 1 study identified by the meta-analysis compared zolpidem and zopiclone. 28 The results reported in this study indicated zolpidem had improved sleep latency, fewer adverse events, and less rebound insomnia. For the comparison between zaleplon and zolpidem, the meta-analysis revealed that zolpidem had more favorable effects on improving quality of sleep compared to zaleplon (OR, 1.51; 95% CI 1.15-1.97; P=.003), but there was no significant difference between the 2 treatments in terms of adverse effects (OR, 0.86; 95% CI, 0.52-1.20). However, analysis of rebound insomnia outcomes data indicated that zaleplon had more favorable effects than zolpidem when the 2 treatments were compared regarding effects on latency to sleep onset (OR, 0.27; 95% CI 0.17-0.44; P<.00001), sleep duration (OR, 0.25; 95% CI 0.15-0.41; P<.00001), and number of awakenings (OR, 0.34; 95% CI 0.18-0.61; P=.0004).
At the present time, 2 additional non-benzodiazepine GABAA receptor agonists are being investigated for the management of insomnia: indiplon (NBI-34060, Neurocrine Biosciences) and gaboxadol (MK-0928, Lundbeck/ Merck). Two separate NDAs were originally filed with FDA in late 2004 for immediate-release and modified-release formulations of indiplon and are awaiting approval.29 In addition, gaboxadol is currently undergoing phase 3 clinical trials. Should these 2 new agents become FDA-approved, they will be added to the existing armamentarium of available non-benzodiazepine receptor agonists. In contrast, ramelteon does not act on GABA receptors; rather, its novel mechanism of action offers an additional approach to the management of insomnia.
SAFETY AND TOLERABILITY Safety data from numerous phase 1/2 studies have been presented as abstracts and posters in various scientific for-ums.16-24,26,27,30 In a study of healthy adult volunteers (N=60), subjects were randomly assigned to receive a single oral dose of either ramelteon (4, 8, 16, 32, or 64 mg) or placebo.19 Performance measures were assessed through the digit-symbol substitution test (DSST) and visual analog scale (VAS) of alertness. Adverse effects, vital signs, electrocardiograms, and standard laboratory tests were recorded to assess for safety measures. Results revealed that cognitive function was not adversely affected by ramelteon at any dose level as measured by mean DSST and VAS scores.
Another phase 1 study by Greenblatt et al assessed ramelteon's effects on performance through the evaluation of DSST and word-list information acquisition and recall tested at intervals up to 24 hours post-dose.22,30 Results from this study indicate that ramelteon did not differ significantly from placebo with regard to its effects on memory (determined via information acquisition and recall). While ramelteon did not appear to affect DSST score for young subjects (male and female, aged 18-34 y) and elderly men (aged 63-79 y), ramelteon's effects in elderly women significantly increased DSST scores compared with placebo (P<.05).
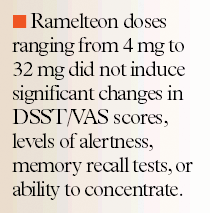
Phase 2 studies have shown that ramelteon doses ranging from 4 mg to 32 mg did not induce significant changes in DSST/VAS scores, levels of alertness, memory recall tests, or ability to concentrate when compared with placebo. However, patients who received the 64-mg dose reported significant decreases in levels of alertness and ability to concentrate (P=.02 and P=.043, respectively).26,27
Numerous studies with small sample sizes (N<60) have shown that the most common side effect was somnolence (up to 50%).19,21,22,31-35 Other reported side effects were nausea, fatigue, dizziness, and headache. In one phase 2 dose-escalation study that included more than 100 subjects with chronic primary insomnia, only mild or moderate adverse effects were observed. Erman et al reported that there were no differences in the number or type of adverse events between any active group (ramelteon 4, 8, 16, 32 mg) and placebo. The most commonly observed adverse events included headache (19.6%), somnolence (7.5%), and pharygnolaryngeal pain (7.5%). None of the subjects exhibited residual next-day pharmacologic effects following ingestion of any dose of ramelteon.27
The most commonly reported adverse events from two phase 2 studies that evaluated doses up to 64 mg were: headache (6.3%-7.1%), somnolence (2.4%-4.8%), nausea (1.6%- 3.3%), and dizziness (0.8%-2.4%).19,26 There were no consistent or clinically significant differences in vital signs, clinical laboratory values, or electrocardiographic findings between the lower- and higher-dose active treatment groups. In addition, subjects did not exhibit any evidence of cognitive impairment.
DRUG INTERACTIONS Ramelteon is not metabolized by any cytochrome p450 (CYP450) isoenzymes. The potential of ramelteon to inhibit or induce those isoenzymes appears to be low as demonstrated in numerous drug interaction studies.31-35
Ramelteon 32 mg once daily coadministered with the CYP3A4 substrate midazolam (10 mg dose),31 dextromethorphan (CYP2D6 substrate) 30 mg once daily for 3 days, or the CYP1A2 substrate32 theophylline 300 mg daily for 10 days,33 did not result in altered pharmacokinetics of any agent. Similarly, concomitant administration of dextromethorphan 30 mg once daily with ramelteon 32 mg once daily did not result in changes in ramelteon plasma exposure.

DOSING AND ADMINISTRATION The specific doses that will be marketed if FDA approval is granted for ramelteon have not been established. One phase 2 trial with ramelteon in patients with primary chronic insomnia (N=107) evaluated doses ranging from 4 mg to 32 mg, administered 30 minutes before habitual bedtime on 2 consecutive nights.27 Doses of 4 mg or greater were shown to reduce latency to persistent sleep to a greater extent than placebo and to increase total sleep time along with sleep efficiency compared with placebo. Ramelteon appeared to exhibit a flat dose-response curve, as shown by a 1.1-minute decrease in mean latency to persistent sleep associated with an 8-fold increase in dose.
A phase 2 trial with ramelteon in healthy volunteers exposed to a novel sleep environment (N=375) evaluated single doses of ramelteon 16 mg and 64 mg, administered 30 minutes before habitual bedtime.26 The results of this study supported the findings of the phase 2 primary chronic insomnia trial, as demonstrated by the 1.5-minute decrease in latency to persistent sleep and the 3-minute increase in total sleep time observed with a 4-fold increase in dose. Of note, the 64-mg dose induced small but statistically significant declines in the ability to concentrate and levels of alertness compared with placebo.
At present, 2 studies have evaluated the effects of single and multiple doses of ramelteon 16 mg in patients with renal and hepatic impairment.23,24 Based on the pharmacokinetic data from the 2 studies, the authors concluded that ramelteon would not require dose adjustment in renally or hepatically impaired patients.
Phase 3 clinical trials have been completed and include data from 7 placebo-controlled trials that evaluated the efficacy of once-daily ramelteon given for various periods of time up to 1 year.10 However, the doses used in the trials are not known, as the data have yet to be released. Based on the phase 2 trials, it appears that doses ranging from 4 mg to 32 mg produce comparable improvements in latency to persistent sleep, total sleep time, and sleep efficiency. Moreover, doses below 64 mg appear to be well-tolerated, as reflected by levels of alertness, concentration abilities, and DSST scores reported by subjects in the morning following ramelteon administration.
REFERENCES 1. Terzano MG, Rossi M, Palomba V, et al. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem, and zaleplon. Drug Safety. 2003;26:261-282.
2. Sateia MJ, Nowell PD. Insomnia. Lancet. 2004;364:1959-1973.
3. Schenck CH, Mahowald MW, Sack RL. Assessment and management of insomnia. JAMA. 2003;289:2475-2479.
4. Sateia MJ, Pigeon WR. Identification and management of insomnia. Med Clin N Am. 2004;88:567-596.
5. Dopheide JA, Stimmel GL. Sleep disorders. In: Koda-Kimble MA, Young LY, eds. Applied Therapeutics: The Clinical Use of Drugs, 8th ed. Philadelphia: Lippincott Williams and Wilkins, 2004:77.1-77.22.
6. Lunesta [package insert]. Marlborough, Mass: Sepracor, Inc; 2005.
7. Sonata [package insert]. Philadelphia, Penn: Wyeth Pharmaceuticals, Inc; 2003.
8. Huckle R. Gaboxadol. Curr Opin Investig Drugs. 2004;5:766-773.
9. News and Articles. New Drug Application submitted for insomnia treatment-indiplon. Available at: http:// http://professionals.epilepsy.com/ newsfeed/pr_1103725813.html. Accessed April 21, 2005.
10. Talk About Sleep. Takeda submits New Drug Application for ramelteon, an investigational sleep drug. Available at: http:// http://www.talkaboutsleep.com/ sleep-disorders/2004/10/insomnia-takeda-drug.htm. Accessed April 21, 2005.
11. Miyamoto M, Nishikawa H, Doken Y, et al. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. 2004;27:1319-1325.
12. Hirai K, Kita M, Ohta H, et al. Ramelteon (TAK-375) accelerates reentrainment of circadian rhythm after a phase advance of the light-dark cycle in rats. J Biol Rhythms. 2005;20:27-37.
13. Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;28:301-310.
14. Hirai K, Kato K, Nishiyama K, et al. TAK-375 and its metabolites are selective agonists at MLs1s receptors [abstract]. Sleep. 2003;26:A79. Abstract 0193.C.
15. Miyamoto M, Kato K, Hirai K, et al. TAK-375 and its active metabolite: lack of binding to non-ML receptor binding sites [abstract]. Sleep. 2003;26:A79. Abstract 0194.C.
16. Stevenson S, Cornelissen K, Clarke E, et al. Study of the absorption, metabolism, and excretion of ramelteon (TAK-375) [abstract]. Clin Pharmacol Ther. 2004;75:P22. Abstract PI-74.
17. Stevenson S, Bryson S, Amakye D, et al. Study to investigate the absolute bioavailability of a single oral dose of ramelteon (TAK-375) in healthy male subjects [abstract]. Clin Pharmacol Ther. 2004;75:P22. Abstract PI-75.
18. Amakye DD, Hibberd M, Stevenson SJ. A phase I study to investigate the absolute bioavailability of a single oral dose of ramelteon (TAK-375) in healthy male subjects [abstract]. Sleep. 2004;27:A54. Abstract 120.
19. Stubbs C, Karim A. A safety, tolerance, and pharmacokinetic study of five single doses of TAK-375 in healthy adults [abstract]. Sleep. 2003;26:A76. Abstract 0187.C.
20. Karim A, Tolbert D, Cao C, et al. Effect of food on the systemic exposure of ramelteon (TAK-375) following a single dose [abstract]. J Clin Pharmacol. 2004;44:1210. Abstract 105.
21. Greenblatt DJ, Harmatz JS. Age and gender effects on the pharmacokinetics of TAK-375 [abstract]. Sleep. 2003;26:A81. Abstract 0198.C.
22. Greenblatt DJ, Harmetz JS, Karim A. Effect of age and gender on the pharmacokinetics of ramelteon (TAK-375), a novel selective ML-1 receptor agonist [abstract]. Presented at: the American Geriatrics Society Annual Meeting; 2004; Las Vegas. Poster 317.
23. Karim A, Tolbert D, Zhao Z. Single and multiple dose pharmacokinetic evaluation of ramelteon (TAK-375) in subjects with and without hepatic impairment [abstract]. J Clin Pharmacol. 2004;44:1210. Abstract 106.
24. Tolbert D, Karim A, Zhao Z. Evaluation of the single and multiple dose pharmacokinetics of ramelteon (TAK-375) in subjects with and without renal impairment [abstract]. J Clin Pharmacol. 2004;44:1210. Abstract 107.
25. Yukuhiro N, Kimura H, Nishikawa H, et al. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Res. 2004;59-66.
26. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303-307.
27. Erman M, Seiden D, Zammit G. Phase II study of the selective ML-1 receptor agonist TAK-375 in subjects with primary chronic insomnia[abstract]. Sleep. 2003;26:A298. Abstract 0748.L.
28. Dundar Y, Dodd S, Strobl J, et al. Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis. Human Psychopharmacol Clin Exp. 2004;19:305-322.
29. Neurocrine Biosciences. Insomnia: indiplon, IR & MR formulations. Available at: http:// http://www.neurocrine.com/html/clin_insomnia.html. Accessed April 21, 2005.
30. Greenblatt DJ, Harmatz JS. Effect of age and gender on the pharmacodynamics of TAK-375: tests of sedation, psychomotor function, and memory [abstract]. Sleep. 2003;26:A80-A81.Abstract 0197.C.
31. Karim A, Tolbert D, Cao C, et al. The effect of multiple doses of ramelteon (TAK-375) on the single-dose pharmacokinetic profile of midazolam in healthy adult subjects [abstract]. Sleep. 2004;27: A47-A48. Abstract 104.
32. Tolbert D, Karim A, Cao C, et al. Study to assess drug interaction between ramelteon (TAK-375) and dextromethorphan in healthy adults [abstract]. Sleep. 2004;27:A50. Abstract 111.
33. Tolbert D, Karim A, Johnson J, et al. Two-period crossover study to assess the drug interaction between ramelteon (TAK-375) and theophylline in healthy adults [abstract]. Sleep. 2004;27:A48. Abstract 105.
34. Karim A, Tolbert D, Cao C, et al. Effects of fluconazole and ketoconazole on the pharmacokinetics of ramelteon (TAK-375) in normal healthy male and female subjects [abstract]. Sleep. 2004;27: A53-A54.
35. Sainati SM, Karim A, Tolbert D. Effects of multiple doses of fluoxetine on the systemic exposure of a single dose of ramelteon (TAK-375) in healthy adults [abstract]. Sleep. 2004;27:A48. Abstract 106.
Dr Nguyen is assistant professor, Pharmacy Practice, University of the Pacific School of Pharmacy, Stockton, Calif, and regional coordinator, Palo Alto Clinical Experience Program, Department of Pharmacy Services, VA Palo Alto Health Care Systems, Palo Alto, Calif. Dr Yu is a clinical pharmacist, Department of Pharmacy Services, Eden Medical Center, Castro Valley, Calif. Dr Song is assistant professor of Pharmacy Practice, University of the Pacific School of Pharmacy, regional coordinator, San Jose Clinical Experience Program, and SCVH&HS pharmacy practice residency coordinator, Department of Pharmacy Services, Santa Clara Valley Medical Center, San Jose, Calif. She can be reached at jessica.song@hhs.co.santa-clara.ca.us
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital in Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
Editors' note: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
