- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Ranolazine: An update on the novel antianginal agent
Ranolazine (Ranexa, CV Therapeutics) is a partial fatty acid oxidase inhibitor that increases the amount of ATP produced from glucose and increases the ability of the myocardium to retain functionality despite a reduced oxygen supply. Ranolazine is under FDA review for the treatment of chronic stable angina (CSA). Ranolazine was first reviewed in the August 2003 issue of Formulary. Since the initial review of ranolazine by FDA, additional data have emerged that merit an update in this journal. Clinical trials have demonstrated the efficacy of ranolazine as both monotherapy and combination therapy in patients with CSA. Recently published clinical trials (MARISA and CARISA) have shown an improvement in symptom-limited exercise duration. The results of the ERICA trial demonstrated a reduction in weekly anginal attacks when ranolazine was added to maximum-dose amlodipine therapy. Headache and generalized weakness were the most commonly reported adverse events in clinical trials. Prolongation of the QT interval has raised concerns; however, a lack of development of ventricular tachyarrhythmias-specifically Torsade de Pointes-remains an important safety finding. (Formulary. 2005;40:323–328.)
Abstract
Ranolazine (Ranexa, CV Therapeutics) is a partial fatty acid oxidase inhibitor that increases the amount of ATP produced from glucose and increases the ability of the myocardium to retain functionality despite a reduced oxygen supply. Ranolazine is under FDA review for the treatment of chronic stable angina (CSA). Ranolazine was first reviewed in the August 2003 issue of Formulary. Since the initial review of ranolazine by FDA, additional data have emerged that merit an update in this journal. Clinical trials have demonstrated the efficacy of ranolazine as both monotherapy and combination therapy in patients with CSA. Recently published clinical trials (MARISA and CARISA) have shown an improvement in symptom-limited exercise duration. The results of the ERICA trial demonstrated a reduction in weekly anginal attacks when ranolazine was added to maximum-dose amlodipine therapy. Headache and generalized weakness were the most commonly reported adverse events in clinical trials. Prolongation of the QT interval has raised concerns; however, a lack of development of ventricular tachyarrhythmias-specifically Torsade de Pointes-remains an important safety finding. (Formulary. 2005;40:323–328.)
Editors' Note: Ranolazine was first reviewed in the August 2003 issue of Formulary (McBride BF, White CM. Focus on ranolazine: A novel metabolic modulator for the treatment of chronic stable angina. Formulary. 2003;38: 461–476). Since the initial review of ranolazine by FDA, additional data have emerged that merit an update in this journal.
An NDA for ranolazine is under review by FDA. In October 2003, the agency issued a letter indicating that ranolazine was approvable, that evidence had suggested it is an effective anti-anginal agent, and that additional clinical information was needed. After a December 2003 Cardiovascular and Renal Drugs Advisory Committee Meeting, CV Therapeutics reached agreement with FDA, under the Special Protocol Assessment (SPA) process, on an approval-enabling study called ERICA (Evaluation of Ranolazine in Chronic Angina). If successful, ERICA could support approval of ranolazine for the treatment of chronic angina in a restricted patient population. The company announced that it had completed enrollment of the ERICA study in November 2004.6,7 If approved, ranolazine will represent the first in a new class of agents for the treatment of CSA and the first new agent for the treatment of CSA in more than 25 years.
This article will focus on recently published clinical trials of sustained-release ranolazine in CSA patients and briefly summarize the other areas. For additional information on the pharmacology of ranolazine and earlier clinical trials, readers can refer to the original "Focus on" article.8
CHEMISTRY AND PHARMACOLOGY
Although the exact mechanism of action of pFOX inhibitors is unknown, ranolazine is thought to work by decreasing fatty acid oxidation and promoting glucose oxidation. Fatty acids are the predominant macronutrient used to generate energy in the myocardium; however, glycogen and glucose can also be used. Fatty acids are preferred because their high-energy chemical bonds yield greater ATP production in normal healthy individuals where oxygen supply is unlimited. All 3 of these macronutrients are used to generate acetyl CoA, which enters the Krebs cycle to produce ATP. ATP provides the energy to power both contractile and non-contractile functions. The selection of macronutrients is regulated by the plasma concentration of fatty acids. That is, the higher the concentration of fatty acids, the less ATP produced via the glycogen and glucose pathways. In patients with CSA, fatty acids are released into the circulation, causing an increase in myocyte uptake, which results in less glucose being utilized as an energy source. However, in patients with CSA and ischemic heart disease, an extra 15% of ATP is produced from glucose oxidation compared to fatty acid oxidation per a standard level of oxygen. If the fatty acid pathway continues to be utilized for the generation of ATP, not enough ATP can be generated and anaerobic metabolism ensues, which is a poor source of ATP generation. By blocking the beta oxidation of fatty acids to acetyl CoA, the hope is that ATP will continue to be generated by the glucose pathway, a more efficient means of energy production in patients with oxygen starved myocytes.9–15
PHARMACOKINETICS
Sustained-release ranolazine has a half-life of approximately 2 hours and reaches peak plasma concentration 4 to 6 hours after ingestion. Ranolazine undergoes significant hepatic metabolism to 11 metabolites (3 major: CVT-2514, representing 33% of parent drug levels at steady state; CVT-2738, representing 27% of parent levels; and CVT-4786, representing 21% of parent levels) with 70% to 75% occurring via the CYP3A isoenzyme system and 10% to 15% via the CYP2D6 pathway. Studies in humans have confirmed that CYP3A4 is the primary metabolic pathway for ranolazine. Less than 5% of ranolazine is excreted unchanged via the kidneys.16–19
CLINICAL TRIALS
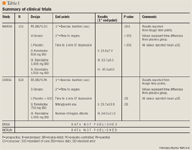
MARISA. The MARISA trial was a randomized, double-blind, multicenter, 4-period crossover trial in which patients with CSA being treated with conventional therapy were withdrawn from their antianginal medications (except for sublingual nitroglycerin as needed) and then treated with sustained-release ranolazine 500, 1,000, or 1,500 mg or placebo twice daily for 1 week.3 The primary efficacy end point was total exercise duration at trough ranolazine concentration (approximately 12 h post-dose) measured via exercise treadmill test (ETT). Secondary end points included time to onset of angina at trough and time to 1-mm ST segment depression at trough. The same measurements were also taken at peak ranolazine concentration (approximately 4 h post-dose). Compared with placebo, treatment with ranolazine improved total exercise duration, time to angina, and time to 1-mm ST segment depression at both trough and peak concentrations (P<.005 for all comparisons). Significant reductions in heart rate or blood pressure were seen only at the 1,500-mg dose. Adverse events were minimal and tended to increase with higher doses of ranolazine. Overall, 168 of the 191 patients enrolled completed all 4 crossover periods. Fifteen of the 23 patients who discontinued the study before completing all treatment periods did so because of adverse events (the specific adverse events that led to study withdrawal were not reported in the trial). One patient died during the study. Minor dose-related increases in the QTc interval were noted; however, no patients withdrew from the study because of QT prolongation and no ventricular tachyarrhythmias were noted (for more information on QT prolongation see the Safety section).
Several subgroup analyses on the primary end point were performed based on history of diabetes, gender, history of New York Heart Association (NYHA) Class I or II heart failure, and age. No significant differences were seen in any subgroup; however, a P value of .054 for gender comparisons is of interest, although not further delineated in the trial. Upon completion of all crossover periods, participants were offered entry into an open-label study evaluating dose escalation and long-term follow-up. Of the 143 patients in the open-label study, 115 (80.4%) and 100 (69.9%) were still on ranolazine therapy at 1- and 2-year time points, respectively. Most of the patients were receiving 1,000 mg twice daily and survival rates were above 90% at each year of follow-up. While this study demonstrated the efficacy of ranolazine on symptoms of CSA, it is important to note some of its limitations. This study had several exclusion criteria: digoxin use, ≥1-mm ST segment depression at rest, left bundle branch block, pacemaker usage, NYHA Class III or IV heart failure, unstable angina, myocardial infarction or coronary revascularization within 2 months, QTc >500 ms or treatment with any agent know to prolong the QT interval, or use of a medication or food known to affect metabolism by CYP3A4. The numerous exclusion criteria and narrow population studied (73.3% male, 91.1% Caucasian, mean age 64.3±9.4 y), limit MARISA's application to the CSA population.
CARISA. The CARISA trial was a prospective, randomized, placebo-controlled, parallel-group trial that evaluated sustained-release ranolazine 750 mg twice daily or 1,000 mg twice daily or placebo in 823 patients with symptomatic CSA who were taking fixed doses of atenolol (354 patients at 50 mg daily), amlodipine (256 patients at 5 mg daily), or diltiazem (213 patients at 180 mg daily).4 The primary end point in this study was ETT duration at trough ranolazine levels (12 h post-dosing) at 2 and 12 weeks of therapy. Time to ischemia, time to angina, angina frequency, and nitroglycerin consumption were also evaluated. Exercise duration improved in patients receiving any dose of ranolazine compared with placebo (P=.01). This effect was independent of drug concentration (peak or trough) and was sustained throughout the completion of the 12-week study. There were no significant differences based on the concomitant anti-anginal agent used (P=.63). Ranolazine reduced the mean number of anginal attacks per week at both the 750-mg and 1,000-mg doses compared with placebo (P=.006 and P<.001, respectively). Nitroglycerin consumption was also reduced at both ranolazine doses (750 mg, P=.02; 1,000 mg, P<.001) after 12 weeks of therapy. Adverse events were mild and similar to those seen in MARISA (eg, dizziness, nausea), and hemodynamic changes remained clinically insignificant. Dose-related increases in the QT interval were seen in patients receiving ranolazine compared with placebo; however, the average overall increase was less than 10 ms. Ranolazine did not affect QT dispersion and no Torsade de Pointes (TdP) was noted. Survival rates in an open-label follow-up study (majority of patients receiving <1,000 mg twice daily) remained above 95% at 2 years. Although this study demonstrated the safe and efficacious combination of ranolazine with traditional antianginal agents, the baseline characteristics and exclusion criteria were similar to the MARISA trial, prompting the need for research in a broader population.
ERICA. The Evaluation of Ranolazine in Chronic Angina (ERICA) trial has been completed, although the results have not been published. This section is lacking in detail due to the paucity of data available solely from a press conference in April 2005.5 Patients received ranolazine in combination with maximum-dose amlodipine and were compared with a control group (control group not further defined) for 6 weeks. Results showed a reduction in weekly anginal attacks by 0.4 in patients who received ranolazine compared to standard of care (this is an assumption based on the data that were vaguely presented at the press conference).5 Secondary end points included a reduction in average weekly nitroglycerin consumption by 0.6 units (presume units=tablets but cannot be certain) and significant improvements in physical activity level in patients receiving ranolazine compared with placebo.5
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
