- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Rational approaches to the treatment of mixed dyslipidemia
This article reviews the role of individual therapeutic agents and combination therapies that can be used for the treatment of mixed dyslipidemia.

Key Points
Abstract
Coronary heart disease causes significant morbidity and mortality. It is estimated that the direct and indirect cost of cardiovascular disease in the United States for 2008 will be $448.5 billion. The widely accepted practice for treating these patients is to target low-density lipoprotein (LDL) cholesterol with optimal statin therapy before considering combination therapy. However, combination therapy is often needed when statin monotherapy does not achieve goals, especially in patients with mixed dyslipidemia, in whom LDL cholesterol and triglyceride levels are elevated and high-density lipoprotein (HDL) cholesterol levels are low. Agents that may be used as add-on therapy to statins include bile acid sequestrants, cholesterol absorption inhibitors, fibrates, nicotinic acid, and omega-3 fatty acids. Selection of a rational combination therapy requires an understanding of the relative efficacy of these agents in addressing individual lipid profile abnormalities and outcomes data. Cholesteryl ester transfer protein (CETP) inhibitors represent a novel class with significant potential in treating low HDL cholesterol levels, an increasingly recognized challenge in hyperlipidemic patients. (Formulary. 2008;43:366–378.)

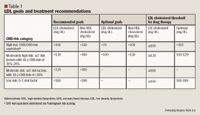

Recent data have brought other cholesterol measures (beyond LDL cholesterol) into focus as therapeutic targets. The risk of CHD-related events has been linked to lower HDL cholesterol levels relative to other lipid panel components such as LDL and total cholesterol.9 Lower HDL cholesterol levels have been associated with an increased risk for CHD.10 Even in patients with low LDL cholesterol secondary to statin treatment, HDL cholesterol increases have been correlated with decreases in cardiovascular risk and a regression of coronary atherosclerosis.11,12
The updated NCEP ATP III guidelines advocate using HMG-CoA reductase inhibitors (statins) capable of lowering LDL cholesterol levels by 30% to 40% as first-line therapy in patients at high or moderately high risk to reduce CHD risk. However, despite treatment with these agents, few patients actually reach treatment goals. Often, using a combination of drugs from different classes of lipid-lowering agents is necessary to achieve aggressive treatment goals, especially as the prevalence of metabolic syndrome increases; in these patients, the optimal LDL cholesterol goal is <70 mg/dL, the optimal TG goal is <150 mg/dL, and the optimal HDL cholesterol goal is >40 mg/dL in men and >50 mg/dL in women. Safety, tolerability, and outcomes evidence should be considered when a combination strategy is implemented. Agents that may be used as add-on therapy to statins include bile acid sequestrants, cholesterol absorption inhibitors, fibrates, nicotinic acid, and omega-3 fatty acids.
This article will review the role of the individual therapeutic agents and combination therapies that can be used to develop rational approaches to the treatment of mixed dyslipidemia. New therapies on the horizon will also be discussed.
INDIVIDUAL AGENTS



Rosuvastatin is the latest statin to be approved by FDA; outcomes data for this agent are scarce. In A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID), 507 patients with CHD or advanced atherosclerosis were treated with rosuvastatin 40 mg/d for 24 months. This treatment resulted in a 6.8% reduction in atheroma volume, as measured by intravascular ultrasound (IVUS).28 The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) was designed to assess the efficacy of rosuvastatin 20 mg/d for the primary prevention of cardiovascular events in 15,000 patients (men aged ≥50 years and women aged ≥60 years) with no history of myocardial infarction, stroke, or arterial revascularization; low LDL cholesterol levels (<130 mg/dL); and elevated levels of C-reactive protein (CRP). On March 31, 2008, AstraZeneca announced that the trial was being stopped early because of significant reductions in cardiovascular morbidity and mortality in the rosuvastatin-treated patients.29
Fibric acid derivatives. Despite extensive studies, the mechanism by which fibric acid derivatives cause their lipid-lowering effects remains unclear. It has been suggested that their effects are related to the activation of peroxisome proliferator-activated receptor (PPAR)-alpha.30 By binding to PPAR-alpha and inducing the clearance of TG-rich lipoproteins, fibric acid derivatives reduce TG levels and increase HDL cholesterol levels. Fibric acid derivatives are the most effective agents to reduce TG levels and the second most effective agents to increase HDL cholesterol levels.
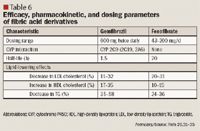
Studies have demonstrated that fibrate therapy reduces CHD morbidity and mortality. In a secondary prevention study called the Veterans Affairs HDL Intervention Trial (VA-HIT), male patients with CHD, low HDL cholesterol, and acceptable LDL cholesterol levels at baseline achieved a 6% increase in HDL cholesterol and a 22% decrease in the relative risk for major coronary events after treatment with gemfibrozil.35 In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, 9,795 patients with type 2 diabetes who were treated with fenofibrate 200 mg/d for 5 years experienced a 24% reduction in nonfatal myocardial infarction and a 21% reduction in coronary revascularization. Overall, this demonstrated that fenofibrate has positive cardiovascular benefits in patients with type 2 diabetes.36
The most common adverse events associated with these agents include gastrointestinal symptoms, such as dyspepsia, nausea, vomiting, and diarrhea. Vigilant monitoring for myositis or rhabdomyolysis is warranted when fibrates are used concurrently with statins. Clinical outcome studies have demonstrated that the incidence of myopathy or rhabdomyolysis is higher in patients receiving gemfibrozil than in patients receiving fenofibrate, either as monotherapy or in combination with a statin.37,38 On the other hand, gemfibrozil is associated with a lower risk of hepatotoxicity compared with fenofibrate.37 ALT and AST monitoring should be regularly performed for patients treated with fibric acid derivatives.
Nicotinic acid. Niacin is the most effective antilipidemic agent for increasing HDL cholesterol levels.39 Niacin increases the levels of apolipoprotein A-1 through elevations in specific lipoprotein particles, which subsequently increases the HDL2 subfraction. The recirculation of these particles leads to HDL level maintenance. Niacin is also involved in partial inhibition of free fatty acid mobilization from the adipose tissue. As a result, the rate of TG removal is increased, leading to decreased levels of TG in the body. LDL cholesterol levels are also affected through this mechanism, as niacin works to decrease the synthesis rate of both very low-density lipoprotein (VLDL) cholesterol and LDL cholesterol in the liver.40,41

Because the NAM pathway is a high-affinity, low-capacity pathway, niacin IR quickly saturates that pathway and is predominantly metabolized through the NUA pathway.42 As a result, undesirable adverse effects, including facial and truncal flushing, itching, rash, and headaches, may be observed. To prevent these adverse effects, healthcare professionals should initiate niacin IR therapy at a low dose, which can be slowly titrated up to the desirable dose over a course of several weeks or months. Taking an aspirin 30 minutes before the niacin dose can reduce the incidence of flushing.43 Patients should also be encouraged to take their doses with food.
Niacin SR was developed to reduce the flushing effect that is normally associated with the niacin IR formulation. Because niacin SR releases niacin over a 12-hour period, this formulation is predominantly metabolized by the NAM pathway. However, undesirable adverse effects, including a higher incidence of hepatotoxicity and gastrointestinal intolerance, may be observed.42 Although niacin SR is readily available over the counter (OTC), this formulation should be avoided.
Niacin ER is the most well tolerated of the 3 niacin formulations. This formulation releases niacin over an 8- to 12-hour period. This mechanism is associated with an improved side-effect profile and efficacy equal to that of niacin IR.39 Niacin ER is effectively metabolized by both pathways, leading to a balanced metabolism.42 This ultimately results in less flushing and less risk of hepatotoxicity, making this agent the favored formulation.43 Niacin ER should be taken once daily at bedtime.
Niacin has not generally been recommended in patients with diabetes because of the agent's propensity to cause modest hyperglycemia. The Arterial Disease Multiple Intervention Trial (ADMIT) evaluated niacin versus placebo in 125 patients with diabetes and peripheral arterial disease for up to 60 weeks. After 18 weeks, niacin IR (average dose, ~2.5 g/d) significantly increased HDL cholesterol by 28.9% and 29.3%, decreased TG by 22.7% and 28.4%, and decreased LDL cholesterol by 8.3% and 9% in diabetic and nondiabetic patients, respectively (P<.001 for niacin vs placebo for all). Niacin IR increased glucose levels by 8.7 mg/dL in the diabetic patients and by 6.3 mg/dL in the nondiabetic patients; hemoglobin A1c (HbA1c) was not significantly changed from baseline in both groups.44 This study suggests that niacin is safe and effective in diabetic and nondiabetic patients.

In the Lipid Research Trial (LRC-CPPT), 3,806 patients treated with cholestyramine had a 19% reduction in nonfatal myocardial infarction or CHD-related mortality.49 In the Cholesterol Lowering Atherosclerosis Study (CLAS), the combination of colestipol and niacin was demonstrated to reduce atherosclerosis progression after 2 years of treatment. Percent changes in coronary artery lesions in the treatment group were statistically significant (0.39% diameter stenosis) compared with the placebo group (2.87% diameter stenosis).50 Colesevelam has also been demonstrated to lower glucose levels and has recently received FDA approval for use in glycemic control in patients with diabetes.48
Selective cholesterol absorption inhibitor. Ezetimibe acts by inhibiting cholesterol absorption from the intestinal lumen at the brush border membrane, leading to a decrease in cholesterol delivery to the liver. As a result, cholesterol stores and circulating cholesterol levels are depleted. However, ezetimibe only modestly decreases LDL cholesterol levels (decrease, 18%–22%) and has minor effects on HDL cholesterol (increase, 1%) and TG (decrease, 8%) levels.26
Ezetimibe produces very minor adverse reactions because of its minimal systemic exposure. The reported side effects observed with ezetimibe include diarrhea, arthralgias, cough, and fatigue.51 There have been no reports of increased risk of myopathy, rhabdomyolysis, or hepatotoxicity when this agent is used in conjunction with a statin.52
Purified omega-3 fatty acids. Purified omega-3 fatty acids are made up of different esters, predominantly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These components lower TG levels by inhibiting the esterification of certain fatty acids, thus decreasing the synthesis of TG.53 Omega-3 fatty acids also enhance postprandial chylomicron clearance and stimulate lipoprotein lipase activity. All of these effects contribute to the overall lowering of TG levels in the body.54 Previously available under the brand name Omacor, purified omega-3 fatty acids are now available under the name Lovaza; this formulation contains 465 mg of EPA and 375 mg of DHA. Omega-3 fatty acids in dietary supplements typically contain less EPA and DHA than the levels contained in Lovaza; the content varies from brand to brand. One study demonstrated that in patients with very high TG levels (>500 mg/dL), the mean placebo-subtracted changes in TG, HDL cholesterol, and LDL cholesterol levels were –51.6%, +9.1%, and +49.3%, respectively.53 The adverse effects most commonly observed in clinical trials include taste perversion, back pain, dyspepsia, and rash.53
COMBINATION THERAPIES
Combination therapy is often needed when statin monotherapy does not allow patients to achieve goals, especially in patients with mixed dyslipidemia, in whom LDL cholesterol and TG levels are elevated and HDL cholesterol levels are often low.
Statin plus bile acid sequestrants. A statin may be combined with a bile acid sequestrant for the agents' additive effect on LDL cholesterol reduction. However, because bile acid sequestrants have little effect on HDL cholesterol and may cause an increase in TG levels, this combination is a poor choice for patients with mixed dyslipidemia.55
Statin plus selective cholesterol absorption inhibitors. Combining a statin with a selective cholesterol absorption inhibitor may allow patients to achieve aggressive LDL cholesterol goals with lower doses of the statin, thus lowering the risk of adverse effects associated with statins. Studies have demonstrated no significant interactions when ezetimibe is added to a statin.56 The combination product simvastatin/ezetimibe has the advantage of reducing pill burden, which may help to improve compliance.
A great debate has surfaced over recent findings of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial, in which patients with familial hypercholesterolemia were treated with either simvastatin 80 mg or a combination of simvastatin 80 mg and ezetimibe 10 mg over a 2-year period.57 The primary end point measure of intima-media thickness (IMT) progression did not improve with the intensive combination regimen, despite significant reductions in LDL cholesterol and C-reactive protein in this group (combination group, 0.0111-mm increase; monotherapy group, 0.0058-mm increase; P=.29). These findings have spurred an intense discussion regarding not only the clinical value of ezetimibe, but also the value of surrogate markers themselves. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) study, which is evaluating outcomes data of cardiovascular morbidity and mortality in 18,000 patients, is expected to be completed by 2012 and should help to define the role of ezetimibe and simvastatin in combination.
Before the introduction of simvastatin/ezetimibe, titrating statins to optimal doses before considering other agents was a widely accepted practice, with the support of ample cardiovascular morbidity and mortality outcomes data.58 Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that as first-line treatment, statins should be administered at the maximum tolerated dose or until cholesterol goals are reached.59 Until more definitive data are available, ezetimibe should be reserved for consideration in primary hypercholesterolemia, after statin dose titration has been attempted. However, adding ezetimibe to statins rarely addresses the challenges of mixed dyslipidemia refractory to statin therapy.
Statin plus fibric acid derivatives. Statins are clearly the drug of choice for lowering LDL cholesterol, as are fibrates for lowering TG. HDL cholesterol increases associated with fibrate treatment are second only to those observed with niacin. This provides a rationale for combination therapy with a statin plus a fibrate in patients with mixed dyslipidemia who respond inadequately to either agent alone. However, combination therapy with a statin plus a fibrate is associated with an increased risk of severe myopathy and rhabdomyolysis. A review of 36 clinical trials and 29 case studies, involving a total of 1,674 patients, demonstrated that the incidence of myopathy was 0.12% when patients were treated with a statin plus a fibrate.60 In another study, the rhabdomyolysis reporting rate from the FDA's Adverse Event Reporting System in patients treated with a statin plus a fibrate was analyzed over a 51-month period. The combination of gemfibrozil and any statin had a rhabdomyolysis reporting rate of 87 cases per 1 million prescriptions. The rate for the combination of fenofibrate and any statin was 4.5 cases per 1 million prescriptions. Using fenofibrate instead of gemfibrozil and avoiding high doses of statins when combining fibrates and statins can lessen the risk for myositis and rhabdomyolysis.61 All in all, this small risk of myopathy rarely outweighs the established morbidity and mortality benefits of achieving lipid goals with this combination.
Statin plus nicotinic acid. The addition of niacin to statin therapy is worthy of consideration, especially when HDL cholesterol levels are very low. Although the time required for slow titration of niacin may discourage the use of this agent, the combination of a statin and niacin has been demonstrated to be both safe and effective. When niacin ER was studied in combination with a statin, no clinically significant myositis occurred, and hepatotoxicity has not been observed to occur more often than with statin therapy alone.55 In addition, relative to gemfibrozil, adding niacin to a statin carries a lower risk of myopathy.60
Statin plus omega-3 fatty acids. The addition of omega-3 fatty acids should be considered when TG levels remain high despite optimal statin dose. In one study, the combination of omega-3 fatty acids 4 g/d and simvastatin 40 mg/d in patients with persistent hypertriglyceridemia despite statin therapy resulted in a placebo-subtracted reduction in TG of 23.2% after 8 weeks; there were no reports of myopathy or hepatotoxicity, and there were minimal changes in HDL and LDL cholesterol levels.62 A similar study conducted over a 1-year period also demonstrated no myopathy or hepatotoxicity.63
In the absence of comparative efficacy data among omega-3 fatty acids, fibrates, and niacin in combination with statins, the choice of combination may be based on prescriber preference, HDL cholesterol level, and concerns about tolerability and adverse effects.
AGENTS IN DEVELOPMENT TO INCREASE HDL CHOLESTEROL
Cholesteryl ester transfer protein (CETP) inhibitors belong to a novel drug class; various agents are currently under development by several companies. These agents block a vital step in cholesterol metabolism-the transfer of cholesteryl esters (CE) from HDL to VLDL and LDL. CETP inhibitors bind to the CETP molecule to prevent this transfer of CE. The effects of this inhibition are increased HDL cholesterol levels (by as much as 46% in patients treated with torcetrapib) and decreased LDL cholesterol levels.64 In December 2006, Pfizer halted Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE), a trial studying torcetrapib, because of concerns regarding increased mortality from off-target pharmacology (hypertension), alterations in lipid-exchange lipolysis of HDL, and potential functional changes in HDL generated from torcetrapib.65–67 Because of these concerns, torcetrapib will not be developed further. Nevertheless, additional CETP inhibitors are currently being investigated by other pharmaceutical manufacturers. In an 8-week trial, Merck's phase 2 agent MK-859 (anacetrapib) was associated with a 129% increase in HDL cholesterol and a 38% decrease in LDL cholesterol.68 Roche/Japan Tobacco's phase 3 agent JTT-705 has been demonstrated to increase HDL cholesterol by 34%.69,70 Large-scale human trials are still necessary to study the safety and efficacy of these compounds. CETP inhibitors represent a novel class with significant potential in treating low HDL cholesterol levels, an increasingly recognized challenge in hyperlipidemic patients.
CONCLUSION
CHD causes significant morbidity and mortality. The treatment of mixed dyslipidemia is a complex affair, especially with the recent trend toward more aggressive lipid goals. ATP III guidelines recommend therapeutic lifestyle changes; after these changes are implemented, LDL cholesterol levels should be targeted with optimal statin therapy. Developments from the ENHANCE trial serve as a cautionary tale that not every novel agent is a better agent. For primary hypercholesterolemia refractory to statin therapy, the addition of ezetimibe is well tolerated; however, this combination has resulted in questionable long-term outcomes data. Although bile acid sequestrants are supported by long-term outcomes data, tolerability is an issue with these agents. In patients with mixed dyslipidemia refractory to statin therapy, combination therapy should be considered. If the predominant abnormality after optimal statin therapy is elevated LDL cholesterol and TG levels and low HDL cholesterol levels, fibric acid derivatives and nicotinic acid are rational choices. When an elevated TG level is the only abnormality after optimal statin therapy, fibric acid derivatives, nicotinic acid, or omega-3 fatty acids are all viable options. Once LDL cholesterol and TG levels are brought under control, further risk reduction can be achieved by reaching HDL cholesterol goals. Existing agents work well for reducing LDL cholesterol and TG levels, but these agents are limited in their ability to increase HDL cholesterol. CETP inhibitors are agents in an emerging class that may have the potential to fill this niche. Although torcetrapib was demonstrated to be unsafe in humans, other CETP inhibitors are being investigated to test their efficacy in increasing HDL cholesterol levels. It will be interesting to watch how these newer agents fare in meeting the ultimate objective-reducing the morbidity and mortality of CHD.
Dr Yeh is a disease management clinical pharmacist, Health Plan of San Joaquin, French Camp, California. Dr Shek is an associate professor of pharmacy practice, University of the Pacific, Stockton, California, and director of pharmacy, Health Plan of San Joaquin. Mr Henriques is a PharmD candidate, University of the Pacific.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. American Heart Association. Heart disease and stroke statistics-2008 update. Dallas, Texas: American Heart Association; 2008. http://www.americanheart.org/downloadable/heart/1200082005246HS_Stats%202008.final.pdf. Accessed September 26, 2008.
2. Schober SE, Carroll MD, Lacher DA, Hirsch R. High serum total cholesterol-an indicator for monitoring cholesterol lowering effects: U.S. adults, 2005–2006. NCHS Data Brief No 2. Hyattsville, MD: National Center for Health Statistics. December 2007.
3. Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Maryland. National Heart, Lung, and Blood Institute. 2002.
4. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [erratum in Circulation. 2004;110:763]. Circulation. 2004;110:227–239.
5. Garg A, Simha V. Update on dyslipidemia. J Clin Endocrinol Metab. 2007;92:1581–1589.
6. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes [erratum in N Engl J Med. 2006;354:778]. N Engl J Med. 2004;350:1495–1504.
7. Chen JT, Wesley R, Shamburek RD, Pucino F, Csako G. Meta-analysis of natural therapies for hyperlipidemia: Plant sterols and stanols versus policosanol. Pharmacotherapy. 2005;25:171–183.
8. Grundy SM, Cleeman JI, Daniels SR, et al; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement [errata in Circulation. 2005;112:e297 and Circulation. 2005;112:e298]. Circulation. 2005;112:2735–2752.
9. Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention trial. Circulation. 2006;113:1556–1563.
10. Luc G, Bard JM, Ferrières J, et al. Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: The PRIME Study. Prospective Epidemiological Study of Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2002;22:1155–1161.
11. Barter P, Gotto AM, LaRosa JC, et al; Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310.
12. Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508.
13. Davidson MH. Overview of prevention and treatment of atherosclerosis with lipid-altering therapy for pharmacy directors. Am J Manag Care. 2007;13(suppl 10):S260–S269.
14. McGinnis B, Olson KL, Magid D, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41:1805–1811.
15. Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90056 participants in 14 randomised trials of statins [errata in Lancet. 2005;366:1358 and Lancet. 2008;371:2084]. Lancet. 2005;366:1267–1278.
16. Altoprev [package insert]. Weston, FL: Andrx Laboratories Incorporated; 2004.
17. Crestor [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2007.
18. Lescol [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2006.
19. Lipitor [package insert]. New York, NY, Pfizer Incorporated; 2007.
20. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Company Incorporated; 2007.
21. Pravachol [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2007.
22. Vytorin [package insert]. North Wales, PA: Merck/Schering-Plough Pharmaceuticals; 2008.
23. Zocor [package insert]. Whitehouse Station, NJ: Merck & Company Incorporated; 2008.
24. Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study) [erratum in Am J Cardiol. 1998;82:128]. Am J Cardiol. 1998;81:582–587.
25. Jones PH, Davidson MH, Stein EA, et al; STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003; 92:152–160.
26. Facts & Comparisons. http://www.factsandcomparisons.com/. Accessed September 24, 2008.
27. Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI Clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:567–572.
28. Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA. 2006;295:1556–1565.
29. Crestor outcomes study JUPITER closes early due to unequivocal evidence of benefit [press release]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; March 31, 2008.
30. Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor-alpha activators. Hypertension. 2005;46:1086–1092.
31. Lopid [package insert]. Morris Plains, NJ: Parke-Davis; 2001.
32. Tricor [package insert]. North Chicago, IL: Abbott Laboratories; 2001.
33. Triglide [package insert]. Atlanta, GA: Sciele Pharma Inc; 2008.
34. Gemfibrozil. In: McEvoy GK, Snow EK, Kester L, Miller J, Welsh OH, Litvak K, eds. AHFS Drug Information 2006. Maryland: American Society of Health-System Pharmacists; 2006.
35. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418.
36. Keech A, Simes RJ, Barter P; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomized controlled trial [errata in Lancet. 2006;368:1415 and Lancet. 2006;368:1420]. Lancet. 2005;366:1849–1861.
37. Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94:935–938.
38. Holoshitz N, Alsheikh-Ali AA, Karas RH. Relative safety of gemfibrozil and fenofibrate in the absence of concomitant cerivastatin use. Am J Cardiol. 2008;101:95–97.
39. McKenney J. Niacin for dyslipidemia: Considerations in product selection. Am J Health Syst Pharm. 2003;60:995–1005.
40. Niaspan [package insert]. Cranbury, NJ: Kos Pharmaceuticals Incorporated; 2007.
41. Kamanna VS, Kashyap ML. Nicotinic acid (niacin) receptor agonists: Will they be useful therapeutic agents? Am J Cardiol. 2007;100:S53–S61.
42. Pieper JA. Understanding niacin formulations. Am J Manag Care. 2002;8(12 suppl):S308–S314.
43. Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007; 99:22C–31C.
44. Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: The ADMIT study: A randomized trial. JAMA. 2000;284:1263–1270.
45. Choi BG, Vilahur G, Viles-Gonzalez JF, Badimon JJ. The role of high-density lipoprotein cholesterol in atherothrombosis. Mt Sinai J Med. 2006;73:690–701.
46. Questran [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1993.
47. Colestid [package insert]. New York, NY: Pfizer Incorporated; 2006.
48. Welchol [package insert]. Parsippany, NJ: Daiichi Sankyo Incorporated; 2008.
49. The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374.
50. Mack WJ, LaBree L, Liu C, Selzer RH, Hodis HN. Correlations between measures of atherosclerosis change using carotid ultrasonography and coronary angiography. Atherosclerosis. 2000;150:371–379.
51. Zetia [package insert]. North Wales, PA: Merck/Schering-Plough Pharmaceuticals; 2008.
52. Florentin M, Liberopoulos EN, Elisaf MS. Ezetimibe-associated adverse effects: What the clinician needs to know. Int J Clin Pract. 2008;62:88–96.
53. Lovaza [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
54. Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98:27i–33i.
55. Worz CR, Bottoroff M. Treating dyslipidemic patients with lipid-modifying and combination therapies. Pharmacotherapy. 2003;23:625–637.
56. Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JR, Bergman AJ, Alton KB. Ezetimibe: A review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467–494.
57. Kastelein JJP, Akdim F, Stroes ES, et al; ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia [erratum in N Engl J Med. 2008;358:1977]. N Engl J Med. 2008;358:1431–1443.
58. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435.
59. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: Consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524.
60. Shek A, Ferrill MJ. Statin-fibrate combination therapy. Ann Pharmacother. 2001;35:908–917.
61. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120–122.
62. Davidson MH, Stein EA, Bays HE, et al; Combination of Prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367.
63. Durrington PN, Bhatnagar D, Mackness MI, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85:544–548.
64. Brosseau ME, Schaefer EJ, Wolfe ML, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350:1505–1515.
65. Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122.
66. Kastelein JJ. Refocusing on use of cholesteryl ester transfer protein inhibitors. Am J Cardiol. 2007;100:47N–52N.
67. Nissen SE, Tardif JC, Nicholls SJ, et al; ILLUSTRATE Investigators. Effect of torcetrapib on the progression of coronary atherosclerosis [erratum in N Engl J Med. 2007;357:835]. N Engl J Med. 2007;356:1304–1316.
68. Krishna R, Anderson MS, Bergman AJ, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomized placebo-controlled phase I studies. Lancet. 2007;370:1907–1914.
69. de Grooth GJ, Kuivenhoven JA, Stalenhoef AF, et al. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans. A randomized phase II dose-response study. Circulation. 2002;105:2159–2165.
70. Dullart RP, Dallinga-Thie GM, Wolffenbuttel BH, van Tol A. CETP inhibition in cardiovascular risk management: A critical appraisal [erratum in Eur J Clin Invest. 2007;35:434]. Eur J Clin Invest. 2007;37:90–98.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
