- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Reducing cardiovascular risk in patients with type 2 diabetes: management of dyslipidemia
Cardiovascular disease (CVD) remains the leading cause of death in patients with diabetes mellitus, accounting for 50% of all deaths. Dyslipidemia is an important modifiable risk factor in diabetic patients and represents a key area for intervention in these patients. Diabetic patients have a lipid profile characterized by low high-density lipoprotein cholesterol (HDL-C) levels and an increase in triglyceride levels. Diabetics have increased numbers of low-density lipoprotein cholesterol (LDL-C) particles but with a shift to smaller, denser LDL-C particles. The net effect is that patients with type 2 diabetes do not have substantially higher LDL-C concentrations than patients without diabetes.

Key Points
Abstract
Cardiovascular disease (CVD) remains the leading cause of death in patients with diabetes mellitus, accounting for 50% of all deaths. Dyslipidemia is an important modifiable risk factor in diabetic patients and represents a key area for intervention in these patients. Diabetic patients have a lipid profile characterized by low high-density lipoprotein cholesterol (HDL-C) levels and an increase in triglyceride levels. Diabetics have increased numbers of low-density lipoprotein cholesterol (LDL-C) particles but with a shift to smaller, denser LDL-C particles. The net effect is that patients with type 2 diabetes do not have substantially higher LDL-C concentrations than patients without diabetes. Lifestyle modification with reductions in saturated fat, trans fat, and cholesterol intake, weight loss (if indicated), and increased physical activity are initially recommended to improve the lipid profile. In addition, enhanced glycemic control can improve plasma lipid levels, especially in patients with very high triglycerides. Statin therapy should be added to lifestyle therapy, regardless of baseline lipid levels for diabetic patients without overt CVD and for diabetics >40 years who have 1 or more other cardiovascular risk factors. For lower-risk patients without overt CVD <40 years of age, statin therapy should be considered in addition to lifestyle therapy if the LDL-C remains above 100 mg/dL or in those with multiple risk factors. In individuals without overt CVD, the primary LDL-C goal is <100 mg/dL. In individuals with overt CVD, a lower LDL-C goal <70 mg/dL, using a high dose of a statin is an option. If drug-treated patients do not reach their LDL-C targets on maximal tolerated statin therapy, a reduction in LDL-C of ~30% to 40% from baseline is an acceptable alternative goal. Triglyceride levels <150 mg/dL and HDL-C >40 mg/dL in men and >50 mg/dL in women are desirable. If triglyceride and HDL-C targets are not achieved using statins, the use of a statin and other lipid-lowering therapy may be considered, but combination therapy has not been shown to reduce the risk of adverse cardiovascular events to an extent greater than that of a statin alone. (Formulary. 2010;45:124-134.)
Microvascular and macrovascular complications of diabetes contribute substantially to its morbidity and mortality.1 Patients with diabetes have a mortality risk comparable to that of patients with established coronary heart disease (CHD)2 and often have extensive atherosclerosis, even without clinical evidence of CHD.3 In addition, once CHD develops in a patient with diabetes, the prognosis is significantly worse than for a patient without diabetes.4 Cardiovascular disease remains the leading cause of death in patients with diabetes mellitus, accounting for 50% of all deaths.5
DYSLIPIDEMIA IN PATIENTS WITH DIABETES
Elevated concentrations of low-density lipoprotein cholesterol (LDL-C) and reduced concentrations of high-density lipoprotein cholesterol (HDL-C) are independent risk factors for the development of premature CHD.8 The role of triglycerides in the premature development of CHD is less well established. The dyslipidemic pattern in diabetes is complex and consists of quantitative and qualitative changes in lipids and lipoproteins. The pattern of dyslipidemia typically observed in the diabetic patient is strongly and independently associated with atherosclerosis.9
Type 2 diabetes is characterized by insulin resistance, which is associated with excess production of very-low-density lipoproteins (VLDL) that leads to triglyceride enrichment of LDL-C and HDL-C and increased lipolysis.10 This results in the production of small, dense LDL-C and HDL-C particles. Smaller HDL-C particles are catabolized more quickly than larger HDL-C particles, resulting in reduced HDL-C levels. Patients with diabetes have an increased number of LDL-C particles but with a shift to smaller, more dense LDL-C particles. The net effect is that patients with type 2 diabetes do not have substantially higher LDL-C concentrations than patients without diabetes.9 The small denser LDL particles are thought to be more atherogenic than larger more buoyant particles.11 Smaller LDL particles can infiltrate the arterial wall more readily, are more easily oxidized, and do not bind as efficiently to the LDL receptor resulting in impaired hepatic clearance of cholesterol.9
HDL-C and apolipoprotein A-I (the primary lipoprotein in HDL-C) levels are reduced in patients with diabetes.12 HDL-C works by removing excess cholesterol from atherosclerotic-plaque-producing cells. Removal of cholesterol from monocyte-derived macrophages (ie, foam cells) is thought to be an important first step in the prevention of progression of atherosclerotic plaques.13 HDL-C promotes sterol efflux from cells via an ATP-binding cassette transporter G1.14 Glycation of apolipoprotein A-I reduces its ability to remove cholesterol from cells using the G1 transporter. The composition of HDL-C is also altered in diabetes, which may affect its atheroprotective potential. HDL-C particles of different sizes and composition demonstrate different abilities to remove cholesterol from cells.13 HDL isolated from patients with type 2 diabetes has been found to have less efficient anti-atherogenic properties.13
Elevated triglyceride levels have predicted the development of premature CHD in univariate analyses and in a meta-analysis, but become less predictive after adjustments for other risk factors.15 Clear associations between triglyceride concentrations and adverse cardiac outcomes are often obscured, as their levels vary inversely with HDL-C concentrations. It has been suggested that postprandial triglyceride levels may more closely correlate with the development of premature CHD than do fasting levels.16 The atherogenic potential of triglycerides is supported by substantial in vitro evidence. Triglyceride-rich lipoproteins increase inflammation and apoptosis in endothelial cells, increase release of tumor necrosis factor, and increase adhesion receptors in macrophages.9 Increased triglycerides produce lipid accumulation in macrophages. In vitro evidence has demonstrated that reductions in triglyceride-rich lipoprotein concentrations prevented disruption of atherosclerotic plaques in a murine model of diabetes whereas increased levels resulted in impaired arterial compliance.9
TREATMENT STRATEGIES FOR DYSLIPIDEMIA
Both the ADA and the National Cholesterol Education Program (NCEP) Expert Panel recommend lifestyle modification as the first step toward improving the lipid profile in patients with diabetes.7,8 The ADA recommends medical nutrition therapy (MNT), which includes a reduction in saturated fat, trans fat, and cholesterol intake. The NCEP recommends therapeutic lifestyle changes (TLC), which include a diet low in saturated fat and cholesterol, use of plant stanols/sterols, and increased consumption of soluble fiber. In addition, both guidelines emphasize weight reduction and exercise. Triglyceride levels can be decreased and HDL-C levels increased with weight loss and increased physical activity. Lifestyle changes may also produce a more modest lowering of LDL-C. In addition, enhanced glycemic control can improve plasma lipid levels, especially in patients with very high triglycerides.
The basic management strategies for dyslipidemia in diabetes differ in the NCEP and ADA guidelines.7,8 Both the ADA and NCEP guidelines recommend optimization of LDL-C as the primary focus of therapy. Following achievement of the LDL-C goal, NCEP recommends lowering triglycerides and then raising the HDL-C. The ADA guidelines recommend that once the LDL-C goal is achieved, management of triglycerides and HDL-C would be considered optional as evidence documenting the benefit of their treatment is lacking.
The optimal levels of the lipoprotein components recommended in the ADA and NCEP guidelines are generally similar. The optimal LDL-C target in diabetic patients is dependent on the presence or absence of overt cardiovascular disease. The optimal LDL-C is <100 mg/dL in diabetics without cardiovascular disease and <70 mg/dL in diabetics with overt cardiovascular disease. In patients with triglyceride levels >200 mg/dL, non-HDL-C (total cholesterol minus HDL-C) levels should be used as a treatment target. The non-HDL-C goal is always 30 mg/dL greater than the respective LDL-C goal. Normal triglyceride levels are <150 mg/dL, and triglyceride levels >200 mg/dL are considered high. Triglyceride levels >500 mg/dL are considered a risk factor for the development of acute pancreatitis. Normal HDL-C levels are >40 mg/dL in men and >50 mg/dL in women.
STATIN THERAPY
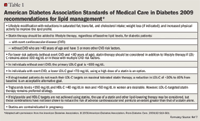
Although patients with diabetes do not typically have markedly elevated LDL-C levels compared with those without diabetes, statins are recommended as first-line therapy for the majority of diabetic patients (Table 1).7 The use of statins in diabetic patients is associated with significant reductions in cardiovascular morbidity and mortality for both primary and secondary prevention. Initial evidence of statin benefit in diabetic patients was derived from post-hoc subgroup analyses from large mortality trials of statins in both primary and secondary prevention.
Secondary prevention trials. In the diabetic substudy of the Scandinavian Simvastatin Survival Study (4S-DM), which included patients with a prior myocardial infarction and elevated total cholesterol levels (212–309 mg/dL), the risk of major coronary events in 483 diabetic patients was reduced by 42% compared with placebo (P=.01).17 This reduction compared favorably to a 32% relative risk reduction in patients with normal fasting glucose and a 38% risk reduction in patients with impaired fasting glucose. In the Cholesterol and Recurrent Events (CARE) trial, 586 diabetic patients with a prior myocardial infarction and average cholesterol levels who were treated with pravastatin had a 25% relative risk reduction compared with placebo (P=.05).18 The magnitude of the risk benefit in the diabetic patients was similar to that of the nondiabetic patients. In the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) trial, which enrolled patients with either a prior myocardial infarction or a hospital admission for unstable angina in the prior 3 to 36 months, pravastatin reduced the risk of cardiovascular events by 21% compared with placebo (P<.008) in a subgroup of 1,077 diabetic patients.19 This compared favorably to a 15% relative risk reduction in the overall study population. In a subgroup analysis of the Treating to New Targets (TNT) trial, atorvastatin 80 mg/d was associated with a 25% reduction in cardiovascular events compared with atorvastatin 10 mg/d in 1,501 diabetic patients with a history of stable CHD.20
Primary prevention trials. A subgroup analysis of the Heart Protection Study (HPS-DM) found a 25% reduction in the risk of major coronary events in 5,963 patients with diabetes.21 This reduction was similar in magnitude to the overall study population. In a further subgroup of 2,912 diabetic patients with no history of cardiovascular disease, simvastatin therapy reduced the risk of coronary events by 33% (P=.0003). This benefit in diabetics without a history of cardiovascular disease was independent of demographics, baseline lipids, duration of diabetes, and glycemic control. The HPS investigators concluded that all diabetes patients should be routinely treated with statins regardless of initial lipid levels.
In a subgroup analysis of the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA) that enrolled hypertensive patients without cardiovascular disease, atorvastatin reduced major cardiac events and procedures by 23% (P=.036) compared with placebo in 2,532 diabetic patients.22 This risk reduction was similar to that achieved in nondiabetic patients.
The Collaborative Atorvastatin Diabetes Study (CARDS) randomly assigned 2,838 diabetes patients with no history of cardiovascular disease but with at least 1 other risk factor (hypertension, current cigarette smoking, retinopathy, or albuminuria) to atorvastatin 10 mg/d or placebo.23 Patients with baseline LDL-C >160 mg/dL and/or triglycerides >265 mg/dL were excluded from the study. The study was terminated 2 years earlier than expected when atorvastatin produced a 37% reduction in major cardiovascular events (P=.001). Atorvastatin reduced LDL-C by 40% compared with placebo. The net benefit of treatment was not altered by demographics, duration of diabetes, glycemic control, blood pressure, smoking, or albuminuria.
The Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN) randomly assigned 2,410 patients with type 2 diabetes to atorvastatin 10 mg/d or placebo.24 Eligible patients had to have a baseline LDL-C ≤160 mg/dL or ≤140 mg/dL if the patient had a prior myocardial infarction or coronary intervention and triglyceride levels <600 mg/dL. Approximately 80% of patients had no history of cardiovascular disease. Baseline LDL-C was approximately 114 mg/dL. After a median follow-up of 4 years, 13.7% of atorvastatin patients and 15% of placebo patients experienced an adverse cardiovascular event (HR=0.90; 95% CI, 0.73–1.12). In patients without a history of cardiovascular disease, 10.4% of atorvastatin patients and 10.8% of placebo patients experienced an adverse cardiac event (HR=0.97; 95% CI, 0.74–1.28). In patients with a history of cardiovascular disease, 26% of atorvastatin patients and 31% of placebo patients experienced a cardiac event (HR=0.82; 95% CI, 0.59–1.15). The relative risk reduction for myocardial infarction was 27% for all patients (P=.10). The relative risk reduction for myocardial infarction in patients without and with prior cardiovascular disease was 19% (P=.41) and 36% (P=.11), respectively.
Summary of statin trials. Post-hoc subgroup analyses of several statin mortality trials indicate that patients with diabetes respond as well as or better than do other study participants for both primary and secondary prevention. The results of these subgroup analyses, however, were not of adequate rigor to drive class I guideline recommendations because diabetes was not typically included as a prespecified treatment subgroup in most of those trials. The HPS-DM and CARDS trials were adequately designed and of sufficient weight to help change the guidelines concerning primary prevention of cardiovascular disease in diabetic patients.21,23
The results of the ASPEN trial indicated that statin therapy does not reduce the risk of adverse cardiovascular events in diabetic patients and stands in direct conflict with the majority of the other outcome studies.24 A number of factors have been cited as reasons for the neutral findings of the ASPEN trial. During the course of the ASPEN study, treatment guidelines for diabetic patients changed, resulting in the discontinuation of the secondary prevention arm of the trial. The drop-in rate of open-label lipid-lowering therapy was 27% in the placebo group compared with 15% in the active treatment group. The baseline lipid profiles would generally not have warranted the use of lipid-lowering therapy (LDL-C 113 mg/dL, HDL-C 47 mg/dL, triglycerides 145 mg/dL). Finally, the relative risk reduction in the atorvastatin group was 27%, a risk reduction as large as most trials associated with statistically significant findings.
The Cholesterol Treatment Trialists' (CTT) Collaborators meta-analysis evaluated 14 randomized outcome trials of statins, which included 18,686 patients with diabetes and 71,370 patients without diabetes.25 The average duration of follow-up in these trials was 4.3 years. There was a 21% relative risk reduction (RR, 0.79; 95% CI, 0.72-0.86; P<.0001) in vascular events for every ~40 mg/dL reduction in LDL-C. This finding was very similar to that observed in nondiabetic patients. All-cause mortality was reduced by 9% (HR=0.91; 95% CI, 0.82–1.01). In studies of primary prevention, the relative risk reduction was 27% (HR=0.73; 95% CI, 0.66–0.82). The results of the CTT meta-analysis and HPS-DM and CARDS trials support the current ADA guidelines indicating that statins should be the first drugs of choice in the management of diabetic dyslipidemia.21,23,25 In patients unable to reach the LDL-C treatment target of <70 mg/dL for diabetic patients with cardiovascular disease or <100 mg/dL for diabetic patients without cardiovascular disease, a 30% to 40% reduction in LDL-C from baseline is considered an acceptable alternate goal.7 The use of a combination of a statin plus another drug that lowers LDL-C, such as ezetimibe, bile acid sequestrants, niacin, or fibric acid derivatives, in order to achieve the guideline-recommended LDL-C, has not been proven to reduce mortality beyond that of a statin alone.
FIBRATE THERAPY
Fibric acid derivatives (fibrates) work by activating the peroxisome proliferator-activated receptor alpha (PPAR-alpha). Activated PPAR-alpha stimulates expression of genes that produce enzymes that regulate fatty acid and lipoprotein metabolism.26 Fibrates promote oxidation of free fatty acids in the liver, which reduces the availability of fatty acids for VLDL synthesis.27 PPAR-alpha stimulation also increases expression of the gene for lipoprotein lipase. Hence, fibrates clear triglycerides from the plasma by both reducing the synthesis and increasing the hydrolysis of triglyceride-rich lipoproteins.26 The reduction in triglyceride levels produces an alteration in the size and composition of LDL-C from small, dense particles to large, buoyant particles.28 The net effect of fibrates on the lipid panel includes a modest reduction in LDL-C concentrations, a modest increase in HDL-C concentrations, and a substantial reduction in triglyceride levels. The changes in lipoprotein levels with fibrates are largely influenced by the patient's baseline lipid levels.29 The higher the baseline LDL-C and triglyceride levels, the greater the net percent reduction in these levels. In addition, the increase in HDL-C levels with fibrates is the greatest in patients with the highest triglyceride levels and the lowest HDL-C levels.
In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial, patients had baseline lipid levels that would have been considered essentially normal and not requiring lipid-lowering therapy.30 In FIELD, fenofibrate monotherapy reduced LDL-C by 18%, increased HDL-C by 4%, and reduced triglycerides by 25%. In studies of other patients with more substantial baseline lipid abnormalities, increases in HDL-C were as high as 20% and reductions in triglycerides were as great as 50%.31 The magnitude of the effect of fibrates on LDL-C is largely dependent on the baseline triglyceride level. In patients with normal triglycerides, the LDL-C is typically reduced by up to 20%. When triglyceride levels are elevated, the effect of fibrates on LDL-C is much more modest and in some cases, LDL-C may actually be increased.
Gemfibrozil. Gemfibrozil has been evaluated in two mortality trials. The Helsinki Heart Study (HHS) was a primary prevention study that enrolled approximately 4,000 middle-aged men with no history of cardiac disease and with non-HDL-C levels >200 mg/dL.32 Gemfibrozil 600 mg twice daily reduced LDL-C by 15 mg/dL and triglycerides by 61 mg/dL and increased HDL-C by 4 mg/dL, while placebo produced minimal changes in these lipid fractions. The cumulative rate of cardiac end points at 5 years was 27.3 per 1,000 in the gemfibrozil group and 41.4 per 1,000 in the placebo group. This represented a 34% decrease in the incidence of CHD (95% CI, 8.2–52.6; P<.02). A subgroup analysis of the HHS, which included only 135 patients with diabetes, demonstrated that gemfibrozil was associated with a lower but statistically insignificant reduction in the development of CHD (3.4% of diabetic patients compared with 10.5% of nondiabetic patients; P=.19).33
The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) was a double-blind, placebo-controlled evaluation of gemfibrozil 1,200 mg/d in 2,531 men with low HDL-C (<40 mg/dL), normal LDL-C levels (<140 mg/dL), and a history of CHD.34 Gemfibrozil produced a substantial reduction in triglycerides (45 mg/dL) but only a minor increase in HDL-C (2 mg/dL). Gemfibrozil also increased LDL-C levels (1.5 mg/dL). Gemfibrozil reduced the risk of the composite end point of cardiovascular death and nonfatal myocardial infarction compared with placebo (relative risk reduction, 22%; 95% CI, 7%–35%), but this end point was driven by a reduction in nonfatal myocardial infarction. Cardiac death was not significantly reduced. In a post-hoc subgroup analysis of 627 diabetic patients in the VA-HIT, gemfibrozil reduced the composite end point by 32% compared with placebo (P=.004), with a reduction of 24% (P<.001) compared to the overall study population.35
Bezafibrate. The Bezafibrate Infarction Prevention (BIP) trial randomly assigned 3,090 CHD patients with elevated total cholesterol levels (180–250 mg/dL) and low HDL (≤45 mg/dL) to bezafibrate (not available in the United States) 400 mg daily or placebo.36 Bezafibrate reduced triglycerides by 21 mg/dL, increased HDL-C by 18 mg/dL, and reduced the LDL-C by 6.5 mg/dL. At the end of an average follow-up of 6 years, the incidence of the composite end point of fatal and nonfatal myocardial infarction and sudden cardiac death was not different between the bezafibrate and placebo groups.
Fenofibrate. Fenofibrate has been studied in 2 mortality trials. FIELD randomly assigned 9,795 diabetic patients to fenofibrate 200 mg/d or placebo.30 Twenty-two percent of the patients had a history of cardiovascular disease at baseline. Mean baseline lipid levels were LDL-C 119 mg/dL, triglycerides 153 mg/dL, and HDL-C 43 mg/dL. Fenofibrate monotherapy decreased LDL-C by 18%, increased HDL-C by 4%, and reduced triglycerides by 25%. A nonsignificant reduction in the combined end point of CHD death and nonfatal CHD was observed in the fenofibrate group (HR=0.89; P=.160). This nonsignificant finding was attributed in part to a low number of adverse cardiovascular events in the placebo group (about 1% per year). In addition, open-label statin use in the placebo group (36%) was higher than that in the fenofibrate group (19%) at study end. Fenofibrate did increase both serum creatinine and homocysteine levels, which may have had an adverse cardiovascular effect. A number of secondary end points were favorably influenced by fenofibrate, including nonfatal myocardial infarction, coronary revascularization, and the microvascular end points of albuminuria and need for retinal laser surgery. However, the statistical relevance of these secondary analyses has been called into question considering the failure to prove the primary study hypothesis. The overall results of FIELD are disappointing given the failure to reduce the primary composite outcome.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) was designed to assess the effect of intensive blood glucose control and either blood pressure or blood lipids on cardiovascular outcomes in patients with type 2 diabetes and an HbA1c >7.5%.37 The intensive blood glucose control arm of the trial was halted prematurely due to an excess mortality rate in the intensive blood glucose treatment group. The lipid arm of the trial included patients aged 40 to 79 years if they had manifest clinical cardiovascular disease. If patients had subclinical cardiovascular disease or at least 2 additional risk factors, age was restricted to 55 to 79 years. Eligible patients had to have LDL-C levels between 60 and 180 mg/dL, an HDL-C less than 55 mg/dL in women and blacks or below 50 mg/dL in all other groups, and a triglyceride level less than 750 mg/dL if patients were not on drug therapy and less than 400 mg/dL if they were receiving lipid therapy.
All patients received open-label simvastatin titrated to achieve guideline-recommended LDL-C levels. One month after initiation of simvastatin, patients were randomly assigned to receive fenofibrate 160 mg/d (n=2,765) or placebo (n=2,753). The mean age of patients was 62 years, 31% were women, 37% had a history of a cardiovascular event, and 60% were taking a statin prior to study enrollment. Baseline lipid levels were an LDL-C of approximately 100 mg/dL, an HDL-C of 38 mg/dL, and a median triglyceride level of 162 mg/dL. The primary composite outcome was the first occurrence of a nonfatal myocardial infarction, nonfatal stroke, or death from a cardiovascular cause.
After a mean follow-up of 4.7 years, 77% of the fenofibrate group remained on therapy, 81% of the placebo group remained on therapy, and 80% of patients in each treatment group remained on simvastatin therapy. An additional 6% of patients were taking an alternative study-approved lipid-lowering agent. At the end of the study, fenofibrate reduced the LDL-C from 100.0 mg/dL to 81.1 mg/dL compared with a reduction from 101.1 mg/dL to 80.0 mg/dL with placebo. Fenofibrate increased the HDL-C from 38.0 mg/dL to 41.2 mg/dL, and HDL-C increased from 38.2 mg/dL to 40.5 mg/dL in the placebo group. Fenofibrate reduced triglycerides from 189.0 mg/dL to 147.0 mg/dL; triglyceride levels were reduced from 186.2 mg/dL to 170.0 mg/dL in the placebo group.
The primary composite end point was not significantly reduced by the addition of fenofibrate to simvastatin when compared with the addition of placebo to simvastatin. In addition, none of the individual components of the primary composite outcome or any secondary outcome was significantly reduced by the use of the combination of fenofibrate and simvastatin compared with simvastatin alone. There was no significant increase in any clinically relevant adverse drug reaction in the combination treatment group compared with the simvastatin alone group, including myopathy or rhabdomyolysis.
In subgroup analyses, men benefited from the use of the combination of fenofibrate and simvastatin, whereas women had evidence of harm. The absolute reduction in cardiovascular events with combination therapy was about 2% in men. Women experienced an absolute increase in cardiovascular events of 2.4%. This gender effect of fenofibrate was not observed in the FIELD trial.30 In a predefined subgroup of patients with baseline triglycerides greater than 204 mg/dL and an HDL-C <34 mg/dL (n=941), the combination of fenofibrate and simvastatin reduced the absolute cardiovascular event rate by 4.95% compared with simvastatin alone (P=.06). This subgroup of patients represented only about 17% of the total study population. The ACCORD LIPID investigators concluded that the addition of fenofibrate to simvastatin does not reduce the risk of fatal cardiovascular events, nonfatal myocardial infarction, or nonfatal stroke compared with simvastatin alone in patients with type 2 diabetes.
Both the FIELD and the ACCORD-LIPID trials suggest that fenofibrate alone or in combination with a statin do not reduce cardiovascular events in type 2 diabetic patients. A major unresolved issue is whether a fibrate would have normally been used as part of a treatment strategy for these patients enrolled in either the FIELD or ACCORD LIPID trials. The baseline lipid levels in both of these studies were not substantially elevated. An argument could be made that the use of fenofibrate alone or in combination with a statin was not indicated according to current treatment guidelines.
Based on the relative paucity of available outcome data, the ADA guidelines do not recommend the routine use of fibric acid derivatives in patients with diabetes.7 In patients with severe hypertriglyceridemia (>500 mg/dL), immediate therapy with lifestyle changes and a fibric acid derivative or niacin is indicated to reduce the risk of acute pancreatitis. In patients without severe hypertriglyceridemia, drug therapy targeting low HDL-C and/or elevated triglycerides is not routinely recommended by the ADA. According to the ADA, gemfibrozil or niacin may be considered in patients with an HDL-C <40 mg/dL and an LDL-C between 100 and 129 mg/dL if they are intolerant to statins.
Niacin. Nicotinic acid (niacin) inhibits lipolysis in adipose tissue, decreases hepatic triglyceride esterification, and increases lipoprotein lipase activity.38 Niacin has been available for 4 decades and is the most effective treatment for raising HDL-C.39 Niacin is a broad-spectrum lipid-lowering agent that lowers LDL-C by an average of 5% to 25%, increases HDL-C by an average of 15% to 35%, and reduces triglycerides by an average of 20% to 50%.8 Niacin increases insulin resistance, and concern remains that it should not be used for lipid management in patients with diabetes or metabolic syndrome.40
The largest clinical end point trial of niacin was the Coronary Drug Project (CDP).41 The CDP enrolled 8,341 men aged between 30 and 64 years with a prior myocardial infarction. Enrollment occurred from March 1966 through October 1969, with a mean treatment follow-up of 6.2 years and a 9-year (total of 15 years) post-treatment follow-up. Patients with diabetes mellitus requiring insulin were excluded from the study. The study was a randomized, placebo-controlled, double-blind comparison of 6 treatment groups, which included conjugated estrogens 2.5 mg/d, conjugated estrogens 5 mg/d, clofibrate 1.8 g/d, dextrothyroxine 6 mg/d, immediate-release niacin 3 g/d, and placebo. Compared with placebo, only niacin reduced cardiovascular events during the 6-year on-treatment follow-up and reduced total mortality at the 15-year follow-up. The number of patients developing dipstick-positive glycosuria or new prescriptions for oral anti-diabetic medications or insulin were not different between the niacin (n=1,119) and placebo (n=2,789) groups. Average postprandial glucose levels increased with the duration of follow-up in both treatment groups, but to a significantly greater extent with niacin. At the end of treatment, the average increase in postprandial blood glucose was 19 mg/mL with niacin (n=542) compared to 9 mg/mL with placebo (P<.001). In a post-hoc analysis of the CDP, niacin produced similar reductions in the risk of myocardial infarction and coronary heart disease deaths at all levels of baseline fasting glucose and at all levels of change in postprandial glucose levels at follow-up.42 In an additional post-hoc analysis of the CDP, 659 niacin-treated patients were identified as having metabolic syndrome.43 Relative risk reductions in myocardial infarction and CHD deaths were not different among niacin-treated patients with and without metabolic syndrome.
Niacin has been shown to slow progression of atherosclerosis when used as monotherapy, in combination with a statin, and in combination with a bile acid sequestrant.44–48 Most of these trials included patients with stable CHD, low HDL-C levels, and normal LDL-C levels. The total numbers of patients enrolled in these trials were relatively small (100–160 patients per study). As a result, the numbers of patients with diabetes included in these trials was also small. In the HDL Atherosclerosis Treatment Study (HATS), 25 of the 160 enrolled patients were diabetics in whom the blood glucose levels following treatment with niacin were higher and fluctuated more widely than in diabetes patients receiving placebo.46 However, changes in progression of atherosclerosis were not adversely affected by the niacin-induced glucose fluctuations. In the Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2 trial, 1 g/d of extended-release niacin or placebo was added to statin therapy in 167 patients with CHD and HDL <45 mg/dL.45 Approximately 25% of patients had diabetes, and 50% had metabolic syndrome. Significant increases in fasting glucose were observed in both the niacin and placebo groups compared to baseline. At the end of 12 months of therapy, carotid intima-media thickness (CIMT) increased significantly with placebo and was unchanged in the niacin group. Although the difference in CIMT progression was not different between the overall niacin and placebo groups, niacin did significantly reduce CIMT progression in patients without insulin resistance (patients without diabetes or metabolic syndrome). These data suggest that niacin is not as clinically effective in patients with insulin resistance.
Two other trials with niacin have been published to further define the extent of glucose intolerance with niacin in patients with diabetes. The Arterial Disease Multiple Intervention Trial (ADMIT) evaluated the effect of niacin on lipid levels in 468 patients with hypercholesterolemia, of which 125 had type 2 diabetes.49 Patients received open-label immediate-release niacin during a 12-week active run-in period, followed by a 48-week double-blind, placebo-controlled treatment period during which niacin was titrated to lipid effect. The average niacin dose was just over 2.5 g/d. Niacin was associated with modest increases in fasting glucose in diabetic patients. Increases in fasting glucose were significantly greater with niacin than with placebo in both patients with and without diabetes. Niacin did not significantly increase HbA1c in diabetes patients. However, diabetic patients receiving placebo had significant reductions in HbA1c. In addition, niacin was not associated with an increased frequency of use of oral antidiabetic drugs or insulin. The percentage of patients discontinuing niacin therapy for glucose intolerance was 6% for patients receiving niacin compared with 3% for those receiving placebo (P=.44). However, 18 patients required niacin dose reductions for excessive HbA1c increases.
The Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial (ADVENT) was a 3-treatment-arm, 16-week study of 148 patients with type 2 diabetes.50 Patients were randomly assigned to extended-release niacin 1 g/d, extended-release niacin 1.5 g/d, and placebo. Niacin 1.5 g/d significantly increased HbA1c from 7.2% to 7.5% (P=.048). Glycemic control worsened in 12% of placebo patients, 18% of niacin 1 g/d patients, and 29% of niacin 1.5 g/d patients. Increased doses of antidiabetic drugs or the addition of a new antidiabetic drug was required in 16% of placebo patients, 24% of niacin 1 g/d patients, and 29% of niacin 1.5 g/d patients. Although the investigators concluded that niacin could safely be used in diabetic patients, the need to add or increase doses of an antidiabetic drug as a result of adding niacin make its choice less than optimal for most diabetes patients.
BILE ACID SEQUESTRANTS
Bile acid sequestrants bind bile acids in the intestine, depleting the bile acid pool and stimulating conversion of cholesterol to bile acids.51 This increases the demand for cholesterol in the liver, which leads to increased hepatic LDL-C receptors. The increase in LDL-C receptors results in increased clearance of LDL-C and decreased LDL-C levels (~15% to 26% reduction). Bile acid sequestrants have a modest effect on HDL-C (3% to 5% increase) and a neutral effect on triglycerides. In patients with elevated triglcyerides, bile acid sequestrants may further increase triglycerides. They are generally not used in patients with baseline triglcyerides >200 mg/dL. The isolated effect of bile acid sequestrants on LDL-C limits their clinical usefulness in diabetes. The clinical role of bile acid sequestrants has typically been in addition to statins in patients who do not achieve their LDL-C targets on maximal dose statins, for patients intolerant to statins, or for patients with contraindications to statins (such as pregnant women).8
The bile acid sequestrants (cholestyramine) have been shown to reduce the risk of CHD, death, and nonfatal myocardial infarction in a primary prevention trial (Lipids Research Clinics Coronary Primary Prevention Trial) in 3,806 patients with LDL-C >190 mg/dL.51 Cholestyramine was associated with an average 20% reduction in LDL-C. Bile acid sequestrants are not systemically absorbed and are typically limited in side effects to those involving the gastrointestinal tract, primarily constipation. Cholestyramine and colestipol were the most commonly used bile acid sequestrants. These agents were administered in granular form. The dosage of these agents required to reduce LDL-C by ~20% was typically 16 to 32 g/d in divided doses.8 At these dosages, compliance with these agents was poor.
Because of poor compliance, as well as the widespread availability of more potent LDL-C reducing agents (statins) and the introduction of ezetimibe, the use of bile acid sequestrants fell out of favor. With the recent efficacy and safety concerns associated with ezetimibe, the availability of a more recently marketed bile acid sequestrant, colesevelam, has gained some favor. Colesevelam is available as a 625-mg tablet with a recommended daily dose of 6 tablets per day. Colesevelam is also now available as a powder containing 3.75 g/packet that can be mixed in 4 to 8 ounces of water to create an oral suspension that can be administered once a day. As monotherapy, colesevelam reduces LDL-C by about 15%.53 It can be combined with a number of other lipid-lowering drugs including statins, niacin, and fibrates.
One unique clinical finding with colesevelam is that it reduces blood glucose and HbA1c.53 Colesevelam reduces HbA1c by an average of 0.6% to 0.9% when used in combination with sulfonylureas, metformin, or insulin. There are only limited data on using colesevelam in combination with thiazolidinediones. Colesevelam is not indicated for use in type 1 diabetes. The mechanism of action of how colesevelam reduces blood glucose is not known, but may be related to absorption of carbohydrates.53 To date, colesevelam is the only drug indicated for treating both dyslipidemia and type 2 diabetes.
EZETIMIBE
Ezetimibe was originally thought to be a non-absorbable substance that blocks cholesterol transport across the intestinal brush border epithelium, resulting in a modest 15% to 18% reduction in LDL-C with little or no effect on HDL-C or triglycerides.54,55 Ezetimibe is well tolerated, with low rates of drug discontinuation. It can be used as monotherapy or in combination with most other lipid-lowering drugs including statins, niacin, and fibrates.56 As with the bile acid sequestrants, ezetimibe was most commonly used in patients not achieving their LDL-C targets on statins, patients intolerant to statins, or patients with contraindications to statins.
The clinical utility of ezetemibe is presently uncertain. The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial failed to show that the combination of ezetimibe and simvastatin was superior to simvastatin alone in slowing the progression of CIMT in patients with familial hypercholesterolemia.57 CIMT actually progressed more in the combination treatment group than in the simvastatin monotherapy group. This lack of effect occurred despite the fact that the combination group had a substantially greater reduction in LDL-C compared to simvastatin alone (60% vs 40%). The lack of beneficial effect with combination therapy has not been easy to explain. It may be that ezetimibe in fact does not have a clinically relevant ability to slow atherosclerosis. The other potential explanation is that in order to have a clinically relevant effect on CIMT in patients with familial hypercholesterolemia and a baseline LDL-C of 318 mg/dL, more than a 60% reduction from baseline over a longer follow-up period is required.
Another disturbing finding with ezetimibe has been the observation of an increased risk of cancer in the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial.58 In the SEAS trial, patients with aortic stenosis were randomly assigned to simvastatin and ezetimibe (n=944) or placebo (n=926). Active drug treatment reduced LDL-C by 61%, compared with 4% with placebo. Patients were followed for an average of 52 months. The primary end point-the frequency of aortic valve replacement-was not favorably affected by active therapy. The only adverse cardiovascular end point significantly reduced by active treatment was the rate of coronary artery bypass graft surgery. The disturbing finding was a significant increase in the risk of cancer with active treatment compared to placebo (11.1% vs 7.5%; P=.01). The risk of fatal cancer during the trial was of borderline significance with active treatment compared to placebo (4.1% vs 2.5%; P=.05). Because of this risk, an interim analysis of 2 large outcomes trials with ezetimibe and simvastatin were unblinded for cancer risk only.58,59 When the interim analysis cancer data from the 2 larger trials were added to the results of the SEAS, the risk of cancer with ezetimibe and simvastatin was no longer significantly greater than with simvastatin alone (P=.61).
THIAZOLIDINEDIONES
The thiazolidinediones are PPAR-gamma agonists that regulate gene expression after activation by free fatty acids.59,60 PPAR-gamma is expressed primarily in adipose tissue and reduces insulin resistance in the liver, fatty tissue, and skeletal muscle.61 As a consequence, glucose uptake in peripheral tissues is increased while hepatic glucose output decreases, resulting in lower blood glucose. Thiazolidinediones decrease plasma and myocardial free fatty acid concentrations and function as potent antioxidants.62 The currently available PPAR-gamma agonists, pioglitazone and rosiglitazone, were approved for use in combination with other antidiabetic drugs. These drugs were initially well received as they were associated with reasonable reductions in HbA1c and a low risk of hypoglycemia. Despite relatively similar effects on blood glucose levels, pioglitazone and rosiglitazone differ in their effects on lipids. Goldberg et al compared the lipid-lowering effects of pioglitazone and rosiglitazone in 802 patients with type 2 diabetes following 24 weeks of treatment.63 Pioglitazone decreased triglycerides by ~52 mg/dL, while rosiglitazone increased triglycerides by ~13 mg/dL (P<.001). Both drugs increased HDL-C, but the magnitude of change was greater with pioglitazone (5.2 mg/dL) compared with rosiglitazone (2.4 mg/dL). Both drugs also increased LDL-C levels, but the magnitude of change was less for pioglitazone (12.3 mg/dL) compared with rosiglitazone (21.3 mg/dL). In addition, LDL-C particle size was increased to a greater extent with pioglitazone than with rosiglitazone (P=.005).
The thiazolidinediones have been evaluated for their effects on cardiovascular outcomes in type 2 diabetic patients. In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) study, pioglitazone did not significantly reduce the primary endpoint (coronary and vascular deaths).64 However, the secondary composite end point that included all-cause mortality, myocardial infarction, and stroke was significantly reduced. Pioglitazone also significantly increased the risk of edema and heart failure, side effects that have been well established as limitations with the use of thiazolidinediones.
Two studies have demonstrated a favorable effect of pioglitazone on atherosclerosis progression, an effect the investigators linked to favorable changes in HDL-C and triglycerides.65,66 In contrast, a meta-analysis of 26,005 patients enrolled in 42 randomized trials found rosiglitazone increased the risk of myocardial infarction by 43% (P=.03) and the risk of death from cardiovascular causes by 64% (P=.06).67 The meta-analysis was limited by a very low overall rate of events, with <1% of participants suffering an adverse cardiac event. It also appeared that much of the increased risk with rosiglitazone occurred when the drug was used in combination with insulin or with nitrate therapy (patients with concomitant CHD). The Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) trial found that rosiglitazone did not increase the risk of overall cardiovascular morbidity and mortality compared with standard antidiabetic drugs.68 RECORD did confirm, however, an increased risk of heart failure and distal lower limb fractures, primarily in women taking rosiglitazone.
Conclusions concerning the cardiovascular safety of the thiazolidinediones remain to be determined. The weight of currently available evidence suggests that if a thiazolidinedione is chosen to treat hyperglycemia in a diabetic patient, pioglitazone would be preferred on the basis of both a more favorable effect on the lipid profile and its cardiovascular risk profile. Both thiazolidinediones are contraindicated in patients with New York Heart Association class III or IV heart failure.
Potential for Combination Lipid-Lowering Therapy
Despite the evidence that statins substantially reduce the risk of adverse cardiovascular outcomes, they do not eliminate that risk entirely. In the TNT study, for example, 14% of stable CHD patients receiving atorvastatin 80 mg/d
experienced a major adverse cardiac event compared with 18% of patients receiving atorvastatin 10 mg/d.20 Although the relative risk reduction in the frequency of adverse outcomes between the treatment groups was 25% and statistically significant, 14% of patients receiving maximal statin therapy still suffered a major cardiac event.
A post-hoc analysis of the TNT study was performed to evaluate the relationship between HDL-C at the end of the third month of statin treatment, the time to the first major cardiac event, and specific strata of LDL-C levels including levels <70 mg/dL.70 HDL-C levels in patients receiving statins were predictive of major cardiac events when HDL-C was considered as both a continuous variable and when it was stratified into quintiles. The association between HDL-C and risk remained significant even after all other baseline risk factors including LDL-C were considered. In statin patients achieving an LDL-C <70 mg/dL, HDL-C remained predictive of cardiovascular risk. In these patients, the higher the HDL-C was, the lower was the risk of cardiac events.
A post-hoc analysis of the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction (PROVE-IT-TIMI-22) study evaluated the relationship between on-treatment levels of triglycerides and LDL-C and adverse cardiovascular outcomes.71 Low triglyceride levels (<150 mg/dL) were associated with a reduced cardiac risk when compared with higher triglyceride levels in univariate analysis as well as after adjustment for LDL-C and non-HDL-C levels. In patients with LDL-C <70 mg/dL, triglycerides <150 mg/dL remained predictive of lower cardiac risk. The post-hoc analyses of the TNT and the PROVE-IT trials suggest that optimization of HDL-C or triglyceride levels in patients receiving optimally dosed statins have the potential to further reduce residual cardiovascular risk.
The results of the post-hoc analyses of the TNT and PROVE-IT trials suggest that adding drug therapy to statins to impact HDL-C and/or triglycerides have the potential to reduce residual cardiovascular risk. There are multiple published studies evaluating the effects of combinations of drugs on lipid levels, most including a statin plus either a fibrate, niacin, ezetimibe, or a bile acid sequestrant. Perhaps the strongest data supporting the use of a combination of lipid-lowering drugs are the HATS and ARBITER 2 trials, which demonstrated a slowing of progression of atherosclerosis with a statin plus niacin.45,46 HATS showed a reduction in clinical events with a statin and niacin, but was underpowered to prove a mortality benefit. As previously discussed, the ACCORD-LIPID trial failed to demonstrate benefit of adding fenofibrate to a statin compared to statin therapy alone in diabetic patients.37 There was a trend toward a reduction in adverse cardiovascular events in a predefined subgroup of patients with triglycerides ≥204 mg/dl and an HDL-C ≤34 mg/dL (P=.06). An outcomes trial comparing statin alone against statin plus niacin (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes-AIM-HIGH) is currently under way.72 This trial is enrolling patients with established cardiovascular disease and atherogenic dyslipidemia (triglycerides >150 mg/dL and HDL-C <40 mg/dL in men and <50 in women). The ADA guidelines do indicate that if lipid targets are not achieved on maximally tolerated doses of statins, combining a statin with other lipid-lowering therapy may be considered to achieve lipid targets.7 This recommendation is based on expert consensus and randomized trials demonstrating reductions in adverse cardiovascular end points (MI, stroke, death) are currently lacking.7
Dr Campbell is pharmacy research fellow, Creighton University Cardiac Center, Omaha, Neb. Dr Hilleman is professor, Pharmacy Practice, Creighton University School of Pharmacy and Health Professions.
Disclosure Information: Dr Campbell reports no financial disclosures as related to products discussed in this article. Dr Hilleman reports that he is on the speakers' bureau for Abbott Laboratories.
REFERENCES
1. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444.
2. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234.
3. Haffner SM, Agostino RD Jr, Saad MF, et al. Carotid artery atherosclerosis in type-2 diabetic and nondiabetic subjects with and without symptomatic coronary artery disease (The Insulin Resistance Atherosclerosis Study). Am J Cardiol. 2000;85:1395–1400.
4. Miettinen H, Lehto S, Salomaa V, et al. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care. 1998;21:69–75.
5. Cubbon RM, Wheatcroft SB, Grant PJ, et al; Evaluation of Methods and Management of Acute Coronary Events Investigators. Temporal trends in mortality of patients with diabetes mellitus suffering acute myocardial infarction: a comparison of over 3000 patients between 1995 and 2003. Eur Heart J. 2007;28:540–545.
6. Rydén L, Standl E, Bartnik M, et al; Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, prediabetes, and cardiovascular diseases: executive summary. Eur Heart J. 2007;28:88-136.
7. American Diabetes Association. Standards of medical care in diabetes-2009. Diabetes Care. 2009;32 Suppl 1:S13–S61.
8. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497.
9. Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809.
10. Goldberg IJ. Clinical review 124: Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86:965–971.
11. Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192:211–217.
12. Mooradian AD, Haas MJ, Wong NC. Transcriptional control of apolipoprotein A-I gene expression in diabetes. Diabetes. 2004;53:513–520.
13. Gowri MS, Van der Westhuyzen DR, Bridges SR, Anderson JW. Decreased protection by HDL from poorly controlled type 2 diabetic subjects against LDL oxidation may be due to the abnormal composition of HDL. Arterioscler Thromb Vasc Biol. 1999;19:2226–2233.
14. Isoda K, Folco EJ, Shimizu K, Libby P. AGE-BSA decreases ABCG1 expression and reduces macrophage cholesterol efflux to HDL. Atherosclerosis. 2007;192:298–304.
15. Criqui MH, Heiss G, Cohn R, et al. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328:1220–1225.
16. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316.
17. Pyörälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614–620.
18. Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998;98:2513–2519.
19. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357.
20. Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226.
21. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016.
22. Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial-lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151–1157.
23. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
24. Knopp RH, d'Emden M, Smilde JG, Pocock SJ; Aspen Study Group. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278.
26. Fruchart JC, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc). 2006;42:39–64.
27. Fazio S, Linton MF. The role of fibrates in managing hyperlipidemia: mechanisms of action and clinical efficacy. Curr Atheroscler Rep. 2004;6:148–157.
28. Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. 2003;31:1066–1069.
29. Farnier M. Update on the clinical utility of fenofibrate in mixed dyslipidemias: mechanisms of action and rational prescribing. Vasc Health Risk Manag. 2008;4:991–1000.
30. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861.
31. Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2005;45:185–197.
32. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245.
33. Koskinen P, Mänttäri M, Manninen V, Huttunen JK, Heinonen OP, Frick MH. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care. 1992;15:820–825.
34. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Liproprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418.
35. Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs High Density Lipoprotein Intervention Trial (VA-HIT). Arch Intern Med. 2002; 162: 2597–2604.
36. Bezafibrate Infarction Prevention (BIP) Study. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation. 2000;102:21–27.
37. The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetics. N Engl J Med. 2010; published online.
38. Wang W, Basinger A, Neese RA, Christiansen M, Hellerstein MK. Effects of nicotinic acid on fatty acid kinetics, fuel selection, and pathways of glucose production in women. Am J Physiol Endocrinol Metab. 2000;279:E50–E59.
39. Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem. 1955;54:558–559.
40. Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc. 2008;83:470–478.
41. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255.
42. Canner PL, Furberg CD, McGovern ME. Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol. 2006;97:477–479.
43. Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol. 2005;95:254–257.
44. Thoenes M, Oguchi A, Nagamia S, et al. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int J Clin Pract. 2007;61:1942–1948.
45. Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517.
46. Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592.
47. Cashin-Hemphill L, Mack WJ, Pogoda JM, Sanmarco ME, Azen SP, Blankenhorn DH. Beneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-up. JAMA. 1990;264:3013–3017.
48. Blankenhorn DH, Selzer RH, Crawford DW, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery. Two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation. 1993;88:20–28.
49. Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: A randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–1270.
50. Grundy SM, Vega GL, McGovern ME, et al; Diabetes Multicenter Research Group. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med. 2002;162:1568–1576.
51. Insull W Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006;99:257–273.
52. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364.
53. Staels B. A review of bile acid sequestrants: potential mechanism(s) for glucose-lowering effects in type 2 diabetes mellitus. Postgrad Med. 2009;121(3 Suppl 1):25–30.
54. Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos. 2002;30:430–437.
55. Taylor AJ. Given the ENHANCE trial results, ezetimibe is still unproven. Cleve Clin J Med. 2008;75:497–506.
56. Miura S, Saku K. Ezetimibe, a selective inhibitor of the transport of cholesterol. Intern Med. 2008;47:1165–1170.
57. Kastelein JJ, Akdim F, Stroes ES, et al; ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443.
58. Rossebo AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356.
59. Drazen JM, D'Agostino RB, Ware JH, Morrissey S, Curfman GD. Ezetimibe and cancer-an uncertain association. N Engl J Med. 2008;359:1398–1399.
60. Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118.
61. Blaschke F, Takata Y, Caglayan E, Law RE, Hsueh WA. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26:28–40.
62. Samaha FF, Szapary PO, Iqbal N, et al. Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:624–630.
63 Goldberg RB, Kendall DM, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554.
64. Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients wtih type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289.
65. Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573.
66. Davidson M, Meyer PM, Haffner S, et al. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117:2123–2130.
67. NissenSE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356: 2457–2471.
68. Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135.
69. Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. 2003. Circulation. 2003;108:2941–2948.
70. Barter P, Gotto AM, Larosa JC, et al; Treating To New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310.
71. Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E; PROVE IT–TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT–TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–730.
72. O'Brien KD. Plaque Inflammation and Dysfunctional HDL Cholesterol in Participants Receiving Niacin and Statins in the AIM-HIGH Study (The HDL Proteomics Study). ClinicalTrials.gov #NCT00880178. 2009. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00880178?term=AIM-HIGH&rank=1/. Accessed March 15, 2010.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
