- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
A review of the pharmacologic options for the treatment of alcohol dependence
The chronic and excessive use of alcohol adversely affects the healthcare system, work productivity, and familial and social relationships. Alcohol misuse accounts for 85,000 deaths per year in the United States, and the overall financial costs related to alcohol dependence are more than $100 billion annually. The reduction of alcohol misuse can be measured as an increase in the frequency of abstinence or a reduction in the frequency of relapses. The recommendation for alcohol dependence treatment is a combination of psychosocial support therapy and pharmacologic treatment. Currently, there are only 3 FDA-approved agents for the treatment of alcohol dependence: naltrexone, acamprosate, and disulfiram.
Dr Angelini is assistant professor, Massachusetts College of Pharmacy and Health Sciences, Boston, Mass. He also maintains an outpatient psychopharmacology practice with the VA Boston Healthcare System. Dr Brahmbhatt is a resident with Clinical Pharmacy Services in the Commonwealth Medicine Managed Care Residency Program, Worcester, Mass. She plans on continuing her development as a practitioner in the managed care arena post-residency.
Abstract
The chronic and excessive use of alcohol adversely affects the healthcare system, work productivity, and familial and social relationships. Alcohol misuse accounts for 85,000 deaths per year in the United States, and the overall financial costs related to alcohol dependence are more than $100 billion annually. The reduction of alcohol misuse can be measured as an increase in the frequency of abstinence or a reduction in the frequency of relapses. The recommendation for alcohol dependence treatment is a combination of psychosocial support therapy and pharmacologic treatment. Currently, there are only 3 FDA-approved agents for the treatment of alcohol dependence: naltrexone, acamprosate, and disulfiram. The literature suggests that there may be varying expectations for the potential beneficial outcomes associated with each of these medications. Treatment with naltrexone has consistently demonstrated a decrease in overall alcohol consumption and fewer relapses when compared with placebo. Acamprosate has been associated with an improvement in maintaining abstinence and more total days of continuous abstinence compared with placebo. Disulfiram treatment has yielded inconsistent results in reducing alcohol consumption when compared with placebo, and observed administration may be the only way to ensure compliance. In general, since disulfiram treatment has not demonstrated long-term efficacy and because of its risk for causing hepatotoxicity, it is not considered a first-line medication option. Other pharmacologic options with differing effects on neurotransmitters also are being studied. Each agent, with its unique mechanism of action, seems to play a role in treating alcohol dependence, either as monotherapy or as part of polypharmacy. Future research is necessary to help specify which patient characteristics will predict the most effective pharmacologic treatment plan for the management of alcohol dependence. (Formulary. 2007;42:14–31.)

Alcohol (ethanol or EtOH) dependence has been estimated to affect 10% of the American population in their lifetime and more than 5% of Americans in any given year.4 When surveyed about recent drinking behavior, students reported that their rate of binge drinking (≥5 drinks in a single occasion) within the previous 2 weeks was 11.9%, 22.2%, and 27.9% for 8th, 10th, and 12th graders, respectively.5
Currently, the Diagnostic and Statistical Manual of Mental Disorders 4th edition (text revised) (DSM-IV-TR) defines substance dependence as having specific characteristics. These characteristics include greater use of the substance than what was planned, the development of tolerance, a withdrawal syndrome if the substance is stopped abruptly, continued use despite the recognized occurrences of medical, legal, and social consequences, and the inability to cut down the amount of the substance used.6
Many factors are involved in the progression to alcohol dependence, which is also commonly referred to as alcoholism.7 Genetics can predispose a person to appreciate the rewarding effects of alcohol ingestion to a very high degree while minimizing the adverse side effects. Another genetic predisposing factor can be a psychiatric disorder such as anxiety, depression, or schizophrenia.8 Alcohol can temporarily alleviate some of the symptoms of these disorders, and thus people may self-medicate with alcohol rather than seeking medical or psychiatric treatment for the disorder.2 Additionally, environmental or psychosocial factors may encourage early and continued use of alcohol.9 Easy access and social acceptability have a significant impact on frequent and abusive use of alcohol. It is important to note that controlled use of 1 to 2 alcoholic drinks per day has not been demonstrated to be harmful to most people. On the contrary, some studies suggest that there may be cardiovascular benefits to this degree of alcohol use.10 Excessive use, such as binge drinking on weekends, is more likely to lead to a drinking pattern that results in harm.11 A signal that someone may have a drinking problem is the inability to control their alcohol intake to 2 drinks or fewer per day.12
What differentiates a casual alcohol user from an alcoholic is the degree of impact that the alcohol has on a person's life. Being an alcoholic means that alcohol has become the most important part of a person's life. All other rewards and responsibilities are less important than using alcohol. This includes one's job, family obligations, relationships, and physical health. This prioritizing of alcohol as most important generally results in a downward spiral of social and health status. Certainly, one can have an alcohol problem and dependence while maintaining a job and family, but eventually the alcohol use needs slowly overtake all other needs and one becomes addicted, along with the subsequent medical complications.12

Currently, there are 3 FDA-approved agents for the treatment of alcohol dependence: disulfiram, naltrexone, and acamprosate. All of these drugs have differing pharmacologies, and combinations of these agents also have been studied. Although costs are increased initially through drug acquisition, greater savings may be seen as a result of abstinence, such as the avoidance of costs associated with hospitalization.15 Controlled clinical trials, as well as those designed to study the treatment of alcohol dependence in a generalizable way reflecting standard care, have demonstrated cost-effectiveness with acamprosate treatment.15,16 Cost-effectiveness has not been directly studied with naltrexone or disulfiram but may be presumed due to the outcome of reduced harmful alcohol use.
DISULFIRAM
In 1951, disulfiram was the first medication approved by FDA for the treatment of alcoholism. Its principal mechanism of action is to prevent aldehyde dehydrogenase from converting acetaldehyde, a metabolite of ethanol, into acetic acid. As acetaldehyde accumulates in the body, it causes certain physiological effects such as nausea, vomiting, flushing, headache, and hypotension ("disulfiram reactions").7 This effect can be seen with small amounts of alcohol, such as the amount in mouthwash. Studies have demonstrated that disulfiram increases the amount of acetaldehyde 5 to 10 times the amount seen with alcohol ingestion alone.17 This process can last for a week post-ingestion, and the intense aversive event is expected to be enough of a psychological deterrent from future alcohol use.11,13 Disulfiram also has been demonstrated to inhibit dopamine hydroxylase, which allows for increased dopamine.18 This has been hypothesized to have some benefit at reducing cravings, although this has not been demonstrated in clinical trials.
The typical dosing of disulfiram is 250 mg every day, although dosing can range from 125 mg to 500 mg a day.17 Typical side effects include drowsiness or a metallic taste. The disulfiram-alcohol interaction can result in cardiovascular problems. Fainting and arrhythmias have been reported; therefore, this agent is not recommended in patients who have, or are at risk for, ischemic heart disease or arrhythmias. Also, disulfiram does have a significant adverse effect of hepatotoxicity that has resulted in hepatic failure and death.17 A number of drug interactions exist with disulfiram, particularly if the compound contains an alcohol base. Any compound with an alcohol base can result in clinically significant increases in acetaldehyde. Disulfiram reactions have also occurred with metronidazole. Disulfiram has a pharmacokinetic interaction with phenytoin, imipramine, and desipramine. It has been demonstrated to increase the serum levels of these 3 medications due to enzymatic inhibition. The addition of disulfiram in a patient taking warfarin has been demonstrated to increase prothrombin time.17
The demonstrated ability of disulfiram to reduce alcohol use has been inconsistent. A study conducted in 605 veterans compared disulfiram 250 mg, disulfiram 1 mg (a control for the threat of the disulfiram-ethanol reaction), and placebo on abstinence rates.19 The study followed patients for up to 1 year and measured alcohol usage every 2 weeks. The study found no difference amongst the 3 arms in abstinence rates or time to first drink.19
Due to its ability to cause such a significant nausea reaction when combined with alcohol, disulfiram acts as a psychological deterrent.13 However, this deterrent is effective only if the medication has been taken that day. Disulfiram is most useful in high-risk alcohol-dependent persons who have a method for compliance such as a caretaker verifying ingestion.20 Because observed administration may be the only way to ensure compliance with disulfiram, and because of its risk for causing hepatotoxicity, it is not considered a first-line agent for treatment of alcohol dependence.20
NALTREXONE
Naltrexone, an opiate receptor antagonist, has been FDA-approved for the treatment of alcohol dependence since 1994. Naltrexone is a specific antagonist for the mu opiate receptor and this is the mechanism by which it reduces relapse rates and heavy drinking.13 Opiate receptor blockade has been demonstrated to reduce the dopamine (DA) release into the nucleus accumbens (NA) that occurs with highly reinforceable behaviors such as alcohol ingestion. By blunting the reward that occurs with drinking, naltrexone decreases the pleasure derived from alcohol ingestion. This reduction in pleasure results in a reduction in use.21 It is also considered one of the mechanisms by which naltrexone reduces cravings. It is postulated that a small amount of mu receptor stimulation occurs when alcohol-dependent persons are reminded of drinking or "cued" for drinking.22 This cue-induced behavior is blunted by the opiate-blocking effect of naltrexone.

Naltrexone oral is approximately 95% absorbed. Production of the primary metabolite, 6-β-naltrexol, is mediated by dihydrodiol dehydrogenase, an enzyme in the cytosol. The cytochrome P450 system is not involved in naltrexone metabolism.26 The most common side effects are headache, nausea, and dizziness, which occurred 2 to 3 times more frequently with naltrexone versus placebo and at a rate of 7%–15% (headache), 10%–14% (nausea), and 5%–12% (dizziness). Discontinuations due to side effects were greater in the naltrexone groups compared with placebo but overall adherence rates were similar for both groups.27,28
Hepatotoxicity has been reported with naltrexone but this event has been rare in the randomized trials and at a rate of about 0.9%.27,29
In 1992, two 12-week clinical trials were the first to demonstrate that naltrexone was effective in treating alcohol dependence.24,30 In the first study, 97 patients were randomized to either naltrexone 50 mg QD or placebo. Both groups were also assigned to supportive therapy or coping skills therapy. The naltrexone group demonstrated a statistical improvement in abstinence rates, number of drinking days, and time to first relapse compared with placebo.24 In the second study, 70 patients who had undergone alcohol detoxifications were randomized to either placebo or naltrexone 50 mg QD. Patients were also assigned to relapse prevention therapy. Naltrexone-treated patients reported fewer cravings for alcohol than the patients receiving placebo, and only 23% of the naltrexone patients relapsed compared with 54.3% of the patients receiving placebo.30
Because of the positive results demonstrated in these 2 preliminary trials, numerous randomized, double-blind, placebo-controlled studies were conducted, and they have demonstrated naltrexone's efficacy in treating alcohol dependence.31–38 These studies have ranged from 12 weeks to 6 months. Some of the studies included post-treatment follow-up. Efficacy compared with placebo was measured via different outcomes, such as: percent of patients relapsing to heavy drinking (defined as ≥5 drinks per episode), percent remaining abstinent, time to first drink, time to relapse (≥5 drinks per episode), total abstinent days per time period enrolled in the study, overall amount drunk per study period, and patient reports of cravings. Naltrexone was not superior to placebo on all these measures in every study. The efficacy measures that naltrexone most consistently demonstrated a favorable response to compared with placebo were the proportion of patients relapsing into heavy drinking and overall amount drunk per study period. The proportion of patients taking naltrexone and relapsing into heavy drinking before the end of these studies varied considerably and ranged from 8% to 93%.
A 12-week, double-blind, placebo-controlled study of naltrexone 50 mg QD was conducted with relapse rate (>5 drinks per day for men or >4 drinks per day for women) as the primary efficacy measure. There were 101 patients enrolled in each arm. Fewer subjects in the naltrexone group relapsed compared with placebo (27.9% versus 18.8%, P=.05).31
A 12-week, multicenter, randomized, double-blind trial compared naltrexone 50 mg QD to placebo in 175 patients. There was no difference in the primary efficacy measure of time to first heavy drinking episode (defined as ≥5 drinks per day for men and ≥4 drinks per day for women, reported as not statistically significant). In the patients who were >80% compliant (n=35 in both arms), there was a statistically significant reduction in the amount of alcohol consumed (approximately 80 drinks per study period per subject for naltrexone and 160 drinks per study period per subject for placebo, P<.05).32
In a 12-week, double-blind, randomized trial, 131 recently abstinent patients were randomized to receive either naltrexone 50 mg QD or placebo. In the primary outcome measure of time to first relapse (defined as ≥5 drinks per day for men and ≥4 drinks a day for women) naltrexone was more effective than placebo (60 days for naltrexone vs 48 days for placebo, P<.02). Also, naltrexone was superior to placebo in regard to percent of days abstinent (90% vs 82% for naltrexone and placebo, respectively; P<.03) and number of drinks per drinking day (2.5 versus 4.2 for naltrexone and placebo, respectively; P<.01).33
Another 12-week, randomized, double-blind study followed by a 20-week open-label period compared naltrexone 50 mg QD with placebo. One hundred twenty-one subjects were studied, with half the patients enrolled in a coping skills program and the other half enrolled in supportive therapy. The coping skills/naltrexone group had the lowest rate of relapse (defined as ≥5 drinks per day) whereas the coping skills/placebo group had the highest (74% vs 97%, respectively; P=.008). The supportive therapy/naltrexone group was no more effective than placebo.32
A 6-month, double-blind, randomized trial compared naltrexone 50 mg QD (n=56) with placebo (n=62). The groups also were divided into CBT or supportive therapy. The combined naltrexone groups demonstrated a lower percentage of heavy drinking days (defined as ≥5 drinks per day for men and ≥4 drinks a day for women) compared with the combined placebo groups (29% versus 38%, naltrexone vs placebo, respectively; P=.045). The groups did not differ in the number of days when drinking occurred (P=.109).34 The CBT group trended toward better outcomes compared with the supportive therapy group (P=.167).34
Another study (n=128) compared naltrexone 50 mg QD to placebo in patients who were given urge-specific coping skills training and communication skills training during a 2-week partial hospitalization program. Subjects were then treated for 12 weeks with drug or placebo and followed for up to a year. For those patients who were >70% compliant, it was demonstrated that the naltrexone group had fewer members relapsing compared with placebo during the 12 weeks of drug therapy. This result was not statistically significant and naltrexone's numerical superiority was lost after the 12-week medication phase. There was a statistical difference favoring naltrexone in the percentage of heavy drinking days (P<.03) and the amount of alcohol consumed during heavy drinking days, with the naltrexone group averaging 5 drinks per episode and the placebo group averaging 9 drinks per episode (P<.05).35 None of these results was significant in the intent-to-treat (ITT) analysis.35
A meta-analysis conducted in 2004 reviewed 19 studies and a total of 3,205 patients.27 The authors determined that relapse rates were almost twice as frequent in the placebo groups as in the short-term (12-week) naltrexone 50 mg QD groups (OR=0.62; 95% CI, 0.52–0.75; P<.001).27 Other measures of efficacy that favored naltrexone compared with placebo were time to relapse (P<.001), drinking days (P<.001), number of drinks per drinking day (P=.001), abstinent days (P=.01), and total alcohol consumption (P<.001).27 Adherence to study protocol was equal between placebo and naltrexone (P=.5), although naltrexone patients experienced higher rates of gastrointestinal side effects (23.6% vs 15.7% for naltrexone and placebo, respectively; P<.001) and neuropsychiatric side effects (42.6% vs 37.3% for naltrexone and placebo, respectively; P<.0008).27
A review conducted in 2005 compared the results from 27 trials using naltrexone 50 mg QD versus placebo or an active comparator.28 There were 3,048 subjects with 1,810 of them treated with naltrexone. The authors determined that short-term naltrexone treatment (12 weeks) resulted in 36% fewer patients relapsing compared with placebo (RR=0.64; 95% CI, 0.51–0.82).28 They also concluded that 18% more patients were likely to stay in treatment (RR=0.82; 95% CI, 0.70–0.97).28 There is less confidence of the impact of medium (6 months) or long-term (1 year) treatment due to the paucity of data and lack of statistical significance. The addition of psychosocial therapy appears to be an important adjunct to naltrexone treatment.28
There also have been some studies conducted resulting in no statistically significant differences between naltrexone and placebo in regard to overall relapse rates.31,36–40 It should be noted that compliance with medication appears critical for positive outcomes. Statistical significance over placebo is almost always attained if there is at least a 70% compliance rate with treatment.24,32,35,38,41

In June 2006, a long-acting intramuscular (IM) injection formulation of naltrexone was approved by FDA for treatment of alcohol dependence. Dosing for this product is 380 mg IM every 4 weeks. This results in a serum level that is similar to a daily oral dose of approximately 150 to 200 mg.43
A study comparing a 380 mg once-monthly injection of naltrexone with placebo demonstrated a decrease in event rate of heavy drinking by 25% (P<.02). Naltrexone was more effective than placebo in the male group (HR=0.56; 95% CI, 0.41–0.77; P<.001). In the female cohort, there was no statistical difference in efficacy compared to placebo (HR=1.23; 95% CI, 0.85–1.78; P=.28).44 It should be noted that the difference in the gender response may have been due to the small number of female patients recruited for the study, along with specific variables that were not measured such as family history.44
All studies for the naltrexone long-acting injection also included some form of weekly psychosocial treatment.44–46 The efficacy outcomes of the long-acting injectable naltrexone compared with placebo appear to be similar to the daily oral version compared with placebo. Adverse events are consistent with naltrexone oral formulations. Most complaints were gastrointestinal, with nausea and decreased appetite being reported 3 times more frequently than with placebo (P<.001 for both nausea and decreased appetite for naltrexone compared with placebo).44 Drop-outs due to side effects were reported as 9% for naltrexone long-acting injectable and 7% for placebo.43 No head-to-head trials have been published comparing the daily oral version to the once-monthly injection.
ACAMPROSATE
Acamprosate received FDA approval in 2004. Indicated for maintenance therapy of abstinence in individuals who are alcohol-dependent, therapy should be initiated in those who have undergone detoxification and are free from any alcohol consumption.47 Unlike other commonly used alcohol dependency alternatives, the effects of this agent do not involve the intense aversion experienced with concomitant alcohol ingestion as seen with disulfiram, nor the attenuated reinforcing effects of alcohol as observed with naltrexone.48
Acamprosate is a synthetic derivative of the endogenous amino acid homotaurine, which is a structural analog of gamma-aminobutyric acid (GABA) and the amino acid neuromodulator taurine. Although its mechanism of activity is not completely understood, various in vitro and in vivo studies have reported the pharmacologic effects of acamprosate and have hypothesized that it has agonist effects on inhibitory GABA neurotransmission and antagonist effects on excitatory glutamate transmission at N-methyl-D-aspartate (NMDA) receptors.49 Acamprosate appears to be involved in correcting the GABA and glutamate balance that is offset in alcohol-dependent individuals. As a result, acamprosate reduces cravings and consumption of alcohol. This was demonstrated initially in a pharmacodynamic analysis of chronically intoxicated rats that exhibited reduced alcohol intake when acamprosate was administered in a dose-dependent manner.50
The recommended dosing regimen for acamprosate in adults is 666 mg TID taken orally. Since nearly all absorbed acamprosate is renally excreted unchanged, dose reduction is necessary in individuals with moderate renal impairment (CrCl 30–50 mL/min) and it is contraindicated in severe renal impairment (CrCl <30 mL/min). Dose adjustment is not required in hepatic impairment as acamprosate does not undergo metabolism via the liver. Acamprosate is not involved in any cytochrome P450 pathways; therefore, no clinically relevant drug interactions have been identified to date.47
Diarrhea continues to be the most frequent adverse effect with acamprosate therapy (10%–17%). Other common adverse events occurring in less than 10% of acamprosate-treated patients include insomnia, anxiety, depression, nausea, abdominal pain, flatulence, anorexia, dizziness, weakness, pruritis, and diaphoresis.47 Due to the complex nature of alcohol dependency, certain adverse effects experienced while using acamprosate may not be entirely treatment-related and potentially may be symptoms associated with alcohol discontinuation.
Acamprosate is not used to treat any symptoms of acute alcohol withdrawal.47 Acamprosate therapy should be initiated for maintaining abstinence as soon as possible subsequent to detoxification. It is also recommended that an appropriate psychosocial support program be introduced simultaneously to increase the likelihood of compliance and motivation to remain abstinent, such as Alcoholics Anonymous, CBT, individualized counseling, or other types of supportive therapy.

In another randomized, double-blind study, patients with a drinking history of at least 12 months (n=188) were initiated on acamprosate therapy (1,332 mg/d or 1,998 mg/d) or placebo following a 14-day detoxification program for a duration of 90 days. At study end, 36.7% of patients had discontinued treatment. A greater proportion of patients in both acamprosate groups remained abstinent throughout the entire study (Day 90, 41%) compared with the placebo group (15%, P<.001), in this dose-ranging study.52 The psychological dependence scores were significantly lower in the acamprosate groups versus placebo (P<.03), with a higher number of patients reporting no desire for alcohol in both acamprosate groups (up to 58% vs 31% in the placebo group) and a lower proportion of patients with an overwhelming desire to drink in the acamprosate groups (up to 13% vs 22% in the placebo group). The percentages of patients experiencing diarrhea were 43% (acamprosate 1,332 mg/d), 48% (acamprosate 1,998 mg/d), and 39% (placebo). Increased libido was reported more frequently with the higher-dose acamprosate group (7 patients vs no patients in the 1,332 mg/d group and 2 patients in the placebo group; P<.05).
Another randomized, double-blind study investigated the effectiveness of acamprosate dosed at 1,332 mg/d (if <60 kg) or 1,998 mg/d (if ≥60 kg) compared with placebo, each with routine but not standardized counseling for 48 weeks (n=272).53 After 1 year, 41.9% of patients receiving acamprosate and 59.6% receiving placebo had withdrawn from the study. Two ITT approaches for analysis were used in this study: one in which those who missed a visit were considered nonabstinent, or the ITT sample, and the other considered missing data as simply missing in the per protocol sample. The acamprosate group achieved a greater continuous abstinence rate of 44.8% compared with 25.3% with placebo at the completion of treatment (P=.005 in the ITT sample; P=.009 in the per protocol sample). A follow-up period of an additional 48 weeks with no active treatment continued to show abstinence rates in favor of acamprosate over placebo (39.9% vs 17.3%, respectively; P= .003). The most common adverse events were mild-to-moderate diarrhea (10 acamprosate-treated patients, 11 placebo-treated patients) and headache (7 acamprosate-treated patients, 9 placebo-treated patients).
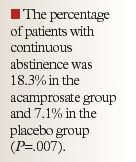
Acamprosate 666 mg TID did not demonstrate a significant difference compared with placebo in the proportion of patients with continuous abstinence at 24 weeks (12% vs 11%, respectively) in a multicenter, randomized, double-blind trial in 581 patients.55 Abstinence was classified as those individuals reporting no drinking on their daily record card, confirmed by negative breath or urine alcohol levels or both. The secondary efficacy variable of cumulative abstinence duration was also not significantly different between acamprosate (77 d) and placebo (81 d; P=.492). However, the additional secondary end point of craving for alcohol, assessed using a visual analog scale, was less in the acamprosate-treated group compared with the placebo-treated group with statistical significance only at Week 2 and Week 4 after treatment initiation (P< .001 vs placebo only at these time points). Of the total enrolled population, 32% had relapsed even before treatment initiation while undergoing detoxification. Only 35% of patients completed the entire 6-month study, with withdrawal mostly due to poor compliance, worsening of condition, or a serious adverse effect. In order to alleviate gastrointestinal symptoms, the total daily dose was reduced from 6 to 4 tablets in 11.4% of patients receiving acamprosate and 9.2% of those receiving placebo.55
An additional randomized, double-blind study of acamprosate (1,332 mg/d if <60 kg or 1,998 mg/d if ≥60 kg) demonstrated no significant differences in continuous abstinence rates when compared with placebo (n=142).56 The study was a short duration period of 8 weeks. The patients in this study received a lower level of psychosocial support and had a history of a more severe degree of alcohol dependence. Premature discontinuation rates were 26.4% (acamprosate) and 31.4% (placebo), with a total of 101 patients (71.1%) completing the study. There were no significant differences in the primary outcome measures of time to first drink (P=.55) or time to relapse, which was defined as days until heavy drinking with ≥5 drinks consumed in a day (P=.90). Another study evaluated the efficacy of acamprosate 1,332 mg/d versus placebo for 90 days.57 This study also had a small sample size (n=26) and at the end of active treatment 7 of the 13 patients in the acamprosate group and 2 out of 13 patients in the placebo group were abstinent (P<.0076). Although an ITT analysis was performed, it is unclear as to whether the 21 patients withdrawing from treatment were truly accounted for.
In another randomized, double-blind, placebo-controlled trial (n=75), 1,998 mg/d of acamprosate was demonstrated to be more effective than placebo, with a greater number of patients remaining continuously abstinent after 12 weeks of treatment followed by 12 weeks without medication [43% (n=17) for acamprosate and 20% (n=7) for placebo; P=.04].58 Participation in Alcoholics Anonymous groups did not appear to favorably affect the mean continuous abstinence time in either group (P=.75). Attrition was similar between groups, with 58 patients (77%) completing the study. Adverse drug events noted more frequently with acamprosate treatment included diarrhea (13% vs 11%), pruritis (10% vs 4%), and headache (10% vs 4%).58
A multicenter, randomized, double-blind study was conducted wherein patients received 1,998 mg/d of acamprosate or placebo for 6 months followed by 3 months without any medication (n=330).59 A post-detoxification program with pharmacotherapy and weekly counseling on alcohol-related problems was also included along with an option for self-help if desired. Approximately 25% of patients withdrew from the study during the first 6 months (40 patients receiving acamprosate and 44 patients receiving placebo). Over the course of treatment the acamprosate group demonstrated a greater abstinence rate compared with placebo; however, a significant difference was not observed until Day 150. Upon completion of the intervention phase, 57.9% of the acamprosate-treated patients were abstinent and 45.2% of the placebo-treated patients were abstinent (P=.03). In the follow-up period, there was a trend toward a continued favorable rate of abstinence with acamprosate, though not significant (P=.154).59 Cumulative abstinence duration over the treatment phase was significantly longer with acamprosate than placebo (P=.016). Frequency of relapse and quantity of alcohol consumption during relapse was lower with acamprosate treatment than placebo at several, but not all, time points (P<.05 for days 30, 60, 150, 180). Although statistically significant, these differences in frequency and quantity during relapse between groups are small and not as relevant clinically. Frequently reported adverse drug reactions were headache (7.3% with acamprosate, 6.6% with placebo), diarrhea (3% with acamprosate, 2.4% with placebo), and epigastric discomfort (1.2% with acamprosate, 5.6% with placebo).59
The majority of the clinical studies consistently demonstrate greater success rates in maintaining continuous alcohol abstinence with acamprosate compared with placebo.51–54,59 In addition, acamprosate has also demonstrated effectiveness in increasing time to first drink and a higher cumulative duration of abstinence compared with placebo.51–54,59 Although the effect of acamprosate on drinking behavior has been evaluated fairly extensively, it has not been studied in individuals with mixed substance abuse.
COMBINATION THERAPY
The simultaneous use of agents with different mechanisms of action in order to achieve a desirable outcome is often a consideration. Currently there are limited data on combination pharmacotherapy of alcohol dependence. Significant pharmacokinetic interactions occur when alcohol dependency medications are co-administered. Naltrexone significantly increases acamprosate levels (approximately 25%), with a more pronounced effect at higher doses. This improved bioavailability seen with the combination of naltrexone and acamprosate may be related to an enhanced clinical effect.60
A recent 4-month, randomized, placebo-controlled trial of multifactorial design (the COMBINE study) enrolled more than 1,300 patients and evaluated the efficacy of naltrexone, acamprosate, behavioral intervention, and combinations thereof in the treatment of newly abstinent individuals with alcohol dependence.61 Patients received either medication management alone (placebo tablets, acamprosate [3 g/d], naltrexone [100 mg/d], or the latter 2 concurrently) or these with combined behavioral intervention (CBI), or CBI alone with no pharmacotherapy. There were 9 study arms. The naltrexone arm was the only intervention that demonstrated a significant effect on time to first heavy drinking day (P=.02), the coprimary outcome measure. The only groups to demonstrate statistical significance in percent of days abstinent were those receiving either naltrexone (80.6%) or CBI (79.2%), with no incremental benefit of combined naltrexone and CBI (77.1%, P=.009 for all). No other combination therapies, including naltrexone with acamprosate, achieved significance in either of the drinking outcome measures.61

An additional trial evaluated combination use of disulfiram and acamprosate (N=110).63 The combination was demonstrated to be more effective in reaching a cumulative abstinence duration (55%, P<.05 vs other subgroups) than either agent used alone (40% for acamprosate vs placebo, P<.05; 31% for disulfiram vs placebo, P value not reported) in the maintenance of abstinence.63
OTHER POTENTIAL THERAPIES
Despite the demonstrated efficacy of naltrexone and acamprosate, there is a need for increased outcomes, particularly on abstinence. the pathology of dependence involves multiple neurotransmitter systems, and there is some recognition that there are subtypes of alcohol-dependent patients that may result in preferential responses to different pharmacotherapies. the type a alcoholic has an onset of alcohol dependence after age 25 with few drinking-related problems and the type b alcoholic has an early age of onset of alcohol use and abuse with significant pathology and strong family history.2This information has helped drive the investigation of other pharmacologic agents for the treatment of alcohol dependence.
One possible group of medications that may play a role are the selective serotonin reuptake inhibitors (SSRIs). However, studies with fluoxetine, sertraline, and citalopram have not demonstrated consistent results at reducing alcohol use in an individual with or without a psychiatric comorbidity. One interesting finding is that the Type B alcoholic seems to have increased rates in drinking in the SSRI group compared with placebo. The Type A alcoholic appears to demonstrate no difference in drinking behavior with an SSRI compared with placebo.64,65 There is a possible relationship between symptoms of anxiety and depression with the use of alcohol, primarily to self-medicate. Until there is a better understanding of alcoholic subtypes, the use of SSRIs for alcohol dependence should be part of a comprehensive treatment plan for an anxious or depressed patient and is not recommended as initial treatment for the alcohol-dependent patient without a psychiatric comorbidity.13
Another agent that works on the serotonergic system, ondansetron, has demonstrated some promise in the treatment of this disorder. In initial studies, ondansetron, a serotonin 3 (5-HT3) receptor antagonist, has demonstrated a reduction in alcohol use among early age of onset (Type B) alcoholics.66

As in many other disorders, the use of multidrug combinations may be considered useful. A number of combination studies using 2 of the 3 agents for alcohol dependence have been conducted. However, these studies have demonstrated conflicting results regarding the potential benefits of combination therapy versus monotherapy.61,69 Other ongoing combination studies are adding naltrexone with some of the previously mentioned agents that are under investigation for this indication.70
CONCLUSION
Excessive alcohol use is harmful to individuals as well as society. Fortunately, our understanding regarding the science of alcohol dependence is rapidly developing. As the environmental risk factors and genetic risk factors are clarified, clinicians will have an enhanced ability to choose the best medication, or combination of medications, for the desired outcome of reduced alcohol use. However, the data at present are not clear enough to give a definitive preferred choice for any particular patient. Both naltrexone and acamprosate have consistently demonstrated efficacy either for reduced relapse rates (naltrexone) or days of cumulative abstinence (acamprosate).27 Disulfiram is reserved for specific patients where witnessed administration is possible as well as no medical comorbidities. Alcohol dependence is a treatment concern for all medical disciplines and a cost concern for all healthcare systems. A mechanism for improved outcomes is likely to involve an increase in clinician education regarding recognition of the disorder and treatment options. These options include availability of various types of psychotherapy for patients and access to alcohol dependence medication.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245 [erratum in JAMA. 2005;293:293–294].
2. National Institute on Alcohol Abuse and Alcoholism. Tenth Special Report to the U.S. Congress on Alcohol and Health. Rockville, Md: US Department of Health and Human Services; 2000.
3. National Institute on Alcohol Abuse and Alcoholism. Estimated economic costs of alcohol abuse in the United States, 1992 and 1998. Available at: http:// http://www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts/EconomicData/cost8.htm.Accessed/ January 5, 2007.
4. Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–473.
5. Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National results on adolescent drug use, overview of key findings, 2003. Bethesda, Md: National Institute on Drug Abuse, 2005.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revised) (DSM-IV-TR). Washington, DC: American Psychiatric Publishing, 2000.
7. Fleming MF, Mihic SJ, Harris RA. Ethanol. In: Brunton LL, Lazo JS, Parker KL, Buxton I, Blumenthal D, eds. Goodman and Gilman's Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill Professional; 2005:602–603.
8. Kessler RC. The epidemiology of dual diagnosis. Biol Psychiatry. 2004;56:730–737.
9. Inaba DS, Cohen WE. Downers: Alcohol. In: Uppers, Downers, and All Arounders. 5th ed. Ashland, Ore: CNS Publications, Inc.; 2004:196–200.
10. Szmitko PE, Verma S. Antiatherogenic potential of red wine: clinician update. Am J Physiol Heart Circ Physiol. 2005;288:2023–2030.
11. US Department of Health and Human Services. Helping patients who drink too much: a clinician's guide. Rockville, Md: US Department of Health and Human Services, 2005.
12. National Institute on Alcohol Abuse and Alcoholism. Alcoholism: getting the facts. Available at: http://pubs.niaaa.nih.gov/publications/GettheFacts_HTML/facts.htm. Accessed January 5, 2007.
13. Mann K. Pharmacotherapy of alcohol depen-dence: a review of the clinical data. CNS Drugs. 2004;18:485–504.
14. Fuller RK, Hiller-Sturmhofel S. Alcoholism treatment in the United States. An overview. Alcohol Res Health. 1999;23:69–77.
15. Poldrugo F, Haeger DA, Comte S, Walburg J, Palmer AJ. A critical review of pharmacoeconomic studies of acamprosate. Alcohol Alcohol. 2005;40:422–430.
16. Rychlik R, Siedentop H, Pfeil T, Daniel D. Cost-effectiveness of adjuvant treatment with acamprosate in maintaining abstinence in alcohol dependent patients. Eur Addict Res. 2003; 9:59–64.
17. Antabuse [package insert]. East Hanover, NJ: Odyssey Pharmaceuticals Inc. 2003.
18. Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin Investig Drugs. 2002;11:491–499.
19. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA. 1986;256:1449–1455.
20. Fuller RK, Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction. 2004;99:21–24.
21. Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59.
22. Monti PM, Rohsenow DJ, Hutchison KE, et al. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394.
23. Weinrieb RM, O'Brien CP. Naltrexone in the treatment of alcoholism. Annu Rev Med. 1997;48:477–487.
24. O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887.
25. Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–292.
26. Revia [package insert]. Wilmington, Del: DuPont Pharma. 1997.
27. Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828 [erratum in: Addiction. 2005;100:573].
28. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.: CD001867.
29. Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. The Naltrexone Usage Study Group. Arch Gen Psychiatry. 1997;54:1130–1135.
30. Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880.
31. Guardia J, Caso C, Arias F, et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26:1381–1387.
32. Chick J, Anton R, Checinski K, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35:587–593.
33. Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–1764.
34. Balldin J, Berglund M, Borg S, et al. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003;27:1142–1149.
35. Monti PM, Rohsenow DJ, Swift RM, et al. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25:1634–1647.
36. Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96:1565–1573.
37. Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176:530–534.
38. Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O'Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742.
39. Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739.
40. Gastpar M, Bonnet U, Boning J, et al. Lack of efficacy of naltrexone in the prevention of alcohol relapse: results from a German multicenter study. J Clin Psychopharmacol. 2002;22:592–598.
41. O'Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13:35–39.
42. O'Malley SS, Jaffe AJ, Chang G, et al. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–224.
43. Vivitrol [package insert]. Cambridge, Mass: Alkermes Inc. 2006.
44. Garbutt JC, Kranzler HR, O'Malley SS, et al; Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625 [erratum in: JAMA. 2005;293:1978; JAMA. 2005;293:2864].
45. Kranzler HR, Wesson DR, Billot L; DrugAbuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004:28:1051–1059.
46. Johnson BA, Ait-Daoud N, Aubin HJ, et al. A pilot evaluation of the safety and tolerability of repeat dose administration of long-acting injectable naltrexone (Vivitrex) in patients with alcohol dependence. Alcohol Clin Exp Res. 2004;28:1356–1361.
47. Campral [package insert]. St Louis, Mo: Forest Pharmaceuticals, Inc. 2004.
48. Doering PL. Substance-related disorders: alcohol, nicotine, and caffeine. In: Pharmacotherapy: A Pathophysiologic Approach. 5th ed. Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Stamford, Conn., Appleton & Lange; 2002:1206–1207.
49. Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: Assessment of the pharmacological actions of acamprosate. Prog Neurobiol. 2000;60:343–362.
50. Le Magnen J, Tran G, Durlach J, Martin C. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol. 1987;4:97–102.
51. Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247.
52. Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997;171:73–77.
53. Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680.
54. Whitworth AB, Fischer F, Lesch OM, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–1442.
55. Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): A 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000;35:176–187.
56. Namkoong K, Lee BO, Lee PG, Choi MJ, Lee E. Acamprosate in Korean alcohol-dependent patients: a multi-centre, randomized, double-blind, placebo-controlled study. Alcohol Alcohol. 2003;38:135–141.
57. Niederhofer H, Staffen W. Acamprosate and its efficacy in treating alcohol dependent adolescents. Eur Child Adolesc Psychiatry. 2003;12:144–148.
58. Baltieri DA, De Andrade AG. Acamprosate in alcohol dependence: A randomized controlled efficacy study in a standard clinical setting. J Stud Alcohol. 2004;65:136–139.
59. Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: A placebo-controlled study. Alcohol Alcohol. 2000;35:202–209.
60. Johnson BA, O'Malley SS, Ciraulo DA, et al. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. J Clin Psychopharmacol. 2003;23:281–293.
61. Anton RF, O'Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: a randomized controlled trial. JAMA. 2006; 295:2003–2017.
62. Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: A double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99.
63. Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: A controlled study. Alcohol Clin Exp Res. 1998; 22:573–579.
64. Kranzler HR, Burleson JA, Korner P, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152:391–397.
65. Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: Interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–1049.
66. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed patients: A randomized controlled trial. JAMA. 2000;284:963–971.
67. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685.
68. Johnson BA, Ait-Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals. Arch Gen Psychiatry. 2004;61:905–912.
69. Kiefer F, Wiedemann K. Combined therapy: What does acamprosate and naltrexone combination tell us? Alcohol Alcohol. 2004;39:542–547.
70. Kenna GA. Pharmacotherapy of alcohol dependence: targeting a complex disorder. Drug Discovery Today: Therapeutic Strategies. 2005;2:71–78.
71. Red Book. 2006 Edition. Montvale, NJ: Thompson PDR; 2006.
72. Red Book Update. October 2006 Edition. Montvale, NJ: Thompson PDR; 2006.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
