- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Rimonabant: A novel CB1 receptor antagonist for the treatment of obesity
Obesity is on the rise in the United States, with 60.5% of the adult population overweight and 23.9% obese as of 2005. Up to 10% of an industrialized country's healthcare budget often can be spent on obesity and associated comorbidities.
Sachin A. Shah, PharmD
Craig I. Coleman, PharmD
C. Michael White, PharmD
Dr Shah is a cardiovascular pharmacology fellow at the School of Pharmacy, University of Connecticut, Storrs, Conn, and Division of Drug Information, Hartford Hospital, Hartford, Conn. Dr Coleman is an assistant professor of pharmacy practice at the School of Pharmacy, University of Connecticut, Storrs, Conn, and director of the Pharmacoeconomics and Outcomes Studies Group at Hartford Hospital, Hartford, Conn. Dr White is an associate professor at the School of Pharmacy, University of Connecticut, Storrs, Conn, and director of the Cardiovascular Pharmacology Service at Hartford Hospital, Hartford, Conn.
Abstract
Obesity over the past 20 years has reached pandemic levels worldwide, with >1 billion people now overweight.1 In 2005, 60.5% of the adult population in the United States was overweight with 23.9% being obese.2 Obesity, alone or in combination with other comorbidities, has been associated with atherosclerotic cardiovascular disease, hypertension, type 2 diabetes, cancer, and respiratory and gallbladder diseases, having a significant toll on patients' health and contributing to increased financial burdens.1 In industrialized countries, 2% to 10% of the total healthcare budget is spent on costs associated with obesity.1 In 2000, the economic cost of obesity was $117 billion in the United States.3
The rising prevalence of obesity and its ramifications have prompted the exploration of several therapeutic strategies including serotonin and noradrenaline inhibitors (sibutramine), lipase inhibitors (orlistat), B3-adrenoreceptor agonists, leptin agonists, and melano-cortin-3 agonists. Meta-analyses of randomized controlled trials with sibutramine and orlistat have demonstrated reductions in weight of 4.5 kg and 2.7 kg, respectively.4, 5 However, their use is limited due to the presence of adverse events and patients' inability to retain weight loss once therapy is discontinued.
One particular observation of cannabis smokers often having hunger pangs led to the development of strategies to block the cannabinoid receptor pathway. Rimonabant (Sanofi-Aventis) is a selective CB1 cannabinoid receptor antagonist approved in Europe as an adjunct to diet and exercise for the treatment of obese or overweight patients with associated risk factors (type 2 diabetes, dyslipidemia).6 In February 2006, rimonabant received an approvable letter from FDA's Division of Metabolism and Endocrinology Products for weight management as an adjunct to diet and exercise and a nonapprovable letter from FDA's Division of Anesthesia, Analgesia and Rheumatology Products for smoking cessation.7 At press time, the developer was continuing to work in collaboration with FDA in resolving the issues required for approval of both NDAs.
CHEMISTRY AND PHARMACOLOGY
The chemical name for rimonabant is 5-[4-chlorophenyl]-1-(2,4-dichlorophenyl)-4-methyl-N-[piperidin-1-yl]-1H-pyrazole-3-carboxamide, and it was initially described by Rinaldi-Carmona et al in 1994.8
Rimonabant is an antagonist of the G-protein coupled CB1 receptor.9 CB1 receptors are located in the areas of the brain responsible for appetite regulation and in several tissues/organs, including adipose tissue, the gastrointestinal tract, liver, skeletal muscle, and autonomic nervous system.10 Hence, rimonabant is expected to have pleiotropic effects other than weight loss.
PHARMACOKINETICS
Rimonabant's pharmacokinetics are relatively dose-proportional up to 20 mg, beyond which area under the curve (AUC) increases are less in proportion to dose.11 Rimonabant is not a P-glycoprotein substrate and displays high in vitro permeability, but the absolute bioavailability has not been determined. In the fasted state, multiple once-daily doses of 20 mg in healthy subjects achieved maximum plasma concentrations of rimonabant in approximately 2 hours, with steady state plasma levels achieved within 13 days. In healthy subjects, Cmax and AUC were increased by 67% and 48%, respectively, underfed compared with fasting conditions. As weight increases from 65 to 200 kg, Cmax is expected to decrease by 24%, and Ctrough to increase by 5%. Peripheral volume of distribution appears to be related to body weight, with obese patients having a higher volume of distribution and taking longer to reach steady state. In vitro, rimonabant exhibits high human plasma protein binding (>99.9%).6
Approximately 3% of the dose is eliminated in the urine, and approximately 86% of the dose is excreted in the feces as unchanged drug and metabolites. Rimonabant is metabolized by CYP3A and amidohydrolase (predominantly hepatic) pathways in which the metabolites are inactive and do not contribute to its pharmacologic effect.6

CLINICAL TRIALS
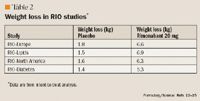
RIO-Europe. RIO-Europe was a multicenter, multinational, double-blind, randomized, placebo-controlled study enrolling 1,507 patients (after 4 weeks' placebo run-in) primarily from Europe.12 Patients with BMI >30 kg/m2 or BMI >27 kg/m2 with comorbid dyslipidemia and/or hypertension received placebo (n=305) or rimonabant 5 mg (n=603) or 20 mg (n=599), in addition to a hypo-caloric diet. Some important exclusion criteria were presence of diabetes mellitus; having significant cardiovascular, hepatic, renal, or pulmonary disorders; or having substantial neurologic or psychiatric illness. Patients with a history of suicide attempts, depression necessitating hospitalization, or a surgical procedure for weight loss (ie, stomach stapling, bypass) also were excluded. Concomitant use of medications that may alter body weight or appetite, including antidepressants, anti-obesity or antidiabetic drugs, corticosteroids, neuroleptics, nicotine substitutes, and nonselective systemic antihistamines, was not permitted, and users of marijuana and hashish were excluded. Average age was 45 years, and average weight was 101 kg. Ninety-four percent of the subjects were white, and 80% were women. Dyslipidemia, hypertension, and metabolic syndrome criteria were met by 61%, 41%, and 41% of the subjects at baseline, respectively. Sixty-one percent of total subjects completed the 1-year follow-up.
In the intent-to-treat analysis using the last observation carried forward method, an average weight reduction of 6.6, 3.4, and 1.8 kg (P<.002 for both rimonabant groups vs placebo) was observed with the rimonabant 20-mg, rimonabant 5-mg, and placebo groups, respectively. Weight loss of at least 5% compared with baseline occurred in 67%, 44%, and 31% of patients receiving rimonabant 20 mg, rimonabant 5 mg, and placebo, respectively; and correspondingly, 39%, 15%, and 12% of the patients lost at least 10% of their weight. Changes in other measured parameters were significant, with improvements in HDL-C (21% vs 12%; P<.001) and triglycerides (–14% vs –1%; P<.001) when comparing rimonabant 20 mg with placebo. Changes in total cholesterol, LDL-C, and blood pressure were not significant. Fasting glucose and insulin reductions were significant but clinically minor.
RIO-Lipids. The randomized, double-blind, placebo-controlled, multicenter, multinational RIO-Lipids study examined the effect of 12 months of rimonabant 20 mg, rimonabant 5 mg, or placebo, in addition to a hypocaloric diet, on body weight in patients who were either overweight or obese, had untreated dyslipidemia, and did not have diabetes.13 Patients aged 18 to 70 years with a BMI of 27 to 40 kg/m2 , fasting plasma triglyceride levels of 1.7 to 1.9 mmol per liter, a total cholesterol to HDL ratio of >5 in men and >4.5 in women, and <5-kg variation in body weight in the previous 3 months were included. Notable exclusion criteria included history of pharmacologic treatment for dyslipidemia within 6 weeks before screening, pharmacologic treatment for weight loss within 3 months before screening, or treatment with a hypocaloric diet within 6 months before screening. Further, patients with diabetes mellitus, systolic or diastolic blood pressure >165 or 105 mmHg, respectively, or histories of clinically significant cardiovascular, endocrine, pulmonary, gastrointestinal, hepatic, or renal disease; marijuana or hashish use; or depression requiring hospitalization (or suicide attempts) also were excluded.
After a 4-week placebo run-in period, 342, 345, and 346 patients were enrolled in the placebo, rimonabant 5-mg, and rimonabant 20-mg groups, respectively. The average age of patients was 48 years, and 40% of patients were male. The average weight of patients was 96 kg. During the 12-month study, about 40% of the patients in each group dropped out, with patient's request being the leading cause for discontinuation in the placebo and rimonabant 5-mg groups, and adverse events the primary reason in the rimonabant 20-mg group. In the intent-to-treat analysis, weight loss of 6.9, 3.1, and 1.5 kg (P<.001 for both rimonabant groups vs placebo) in the rimonabant 20-mg, rimonabant 5-mg, and placebo groups, respectively, was observed. Overall, patients who had weight loss >5% of total body weight accounted for 19.5% of the placebo and 58.4% of the rimonabant 20-mg groups, respectively. In the rimonabant 20-mg and placebo groups, 33% and 7% of patients, respectively, lost at least 10% of their body weight. Other parameters such as HDL-C demonstrated a modest increase (19% vs 11% for placebo; P<.001), whereas triglycerides demonstrated a modest decrease (13% vs no significant change in placebo; P<.001) in the rimonabant 20-mg group. Total cholesterol (1.6%, 2.9%, and 2.3% in rimonabant 20-mg, rimonabant 5-mg, and placebo groups, respectively) and LDL-C (7.2%, 6.6%, and 7.0% in rimonabant 20-mg, rimonabant 5-mg. and placebo groups, respectively) increased in all treatment groups but these increases were not statistically significant. Although systolic and diastolic blood pressure was significantly decreased in the rimonabant 20-mg group, this change was clinically insignificant. Significant reduction in fasting plasma glucose was not observed in any group.

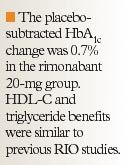
RIO-Diabetes. In this multinational, multicenter study, 1,045 obese or overweight patients with type 2 diabetes were enrolled and randomized to rimonabant 20 mg (n=339), rimonabant 5 mg (n=358), or placebo (n=348).15, 20 Eligible patients had to be taking metformin or a sulfonylurea monotherapy for a minimum of 6 months, with fasting plasma glucose between 100 and 271 mg/dL and HbA1c between 6.5% and 10%. Average weight at enrollment was approximately 98 kg. Mean age of patients was 56 years and nearly half the patients were women. At 1 year, in the intent-to-treat analysis, treatment with rimonabant 20 mg or placebo was associated with a 5.3-kg and a 1.4-kg (P<.001) weight reduction, respectively, whereas the rimonabant 5-mg group experienced a weight reduction of 2.3 kg.20,21 The placebo-subtracted HbA1c change was 0.7% in the rimonabant 20-mg group. HDL-C and triglyceride benefits were similar to previous RIO studies, but more information will be available upon publication of the full report.
STRATUS-US. The STRATUS-US trial evaluated the efficacy of rimonabant in inhibiting nicotine and food cravings.19 Smokers (N=787) with a desire to stop smoking were randomized to placebo, rimonabant 5 mg, or rimonabant 20 mg. Subjects started treatment 2 weeks before stopping smoking and continued treatment for 8 weeks thereafter. A significantly greater number of subjects stopped smoking with rimonabant 20 mg compared with placebo (27.6% vs 16.1%; P=.004). Seventy-seven percent of the subjects taking rimonabant who were not obese at baseline did not have post-cessation weight gain compared with placebo.
More data from this study will be available upon publication of the full report of STRATUS-US.
Several other rimonabant studies (Table 1) are under way22–30 ; some of these have been completed, whereas others are still recruiting.
ADVERSE EVENTS
Treatment-related adverse events reported in >5% of patients in RIO-Lipids included nausea, dizziness, influenza, anxiety, diarrhea, and insomnia occurring more frequently in the rimonabant groups compared with placebo. More patients discontinued treatment due to adverse events in the rimonabant 20-mg group, although overall discontinuation rates were similar in all groups. The most common reasons for discontinuation included depression, anxiety, and nausea.
In the RIO-Europe study, the frequency of adverse events was higher in the rimonabant 20-mg group compared with the other 2 groups. Adverse events with rimonabant in >5% of patients included nausea, dizziness, diarrhea, and arthralgia. Serious adverse events were similar among all groups except for psychiatric disorders.
In the RIO-North America study, at 1 year, the number of patients who reported at least 1 adverse event was similar among all groups. Adverse events, particularly psychiatric-, nervous system-, and gastrointestinal-related, led to study withdrawal in rimonabant-treated patients.

DRUG INTERACTIONS
Metabolism of rimonabant occurs by both CYP3A and amidohydrolase pathways. In 1 study, rimonabant AUC increased by 104% with administration of the CYP3A4 inhibitor ketoconazole. Hence, the use of other CYP3A4 inhibitors (eg, itrazonazole, ritonavir, clarithromycin) concomitantly with rimonabant requires caution. Though not studied, reductions in rimonabant concentrations and a lack of efficacy can be expected with CYP3A4 inducers (eg, rifampin, phenytoin, St. John's wort). Plasma levels of rimonabant are not significantly affected by orlistat, ethanol, or lorazepam.6
In vitro, rimonabant does not induce or inhibit other CYP enzymes or P-glycoproteins. Rimonabant has a mild inhibitory effect on CYP2C8 (in vitro), but the potential for this inhibition in vivo appears low. Rimonabant does not affect steady state pharmacokinetics of ethinyl/levonorgestrel combination oral contraceptives.6
DOSAGE AND ADMINISTRATION
In Europe, rimonabant is marketed as Acomplia and available as a 20-mg film-coated tablet. One 20-mg tablet is recommended to be taken in the morning before breakfast. The safety and efficacy of rimonabant have not been evaluated for periods longer than 2 years. Dose adjustments are not necessary in the elderly. However, caution is advised in patients aged >75 years and aged <18 years due to limited or lack of available data. Dose adjustments are also not required for mild-to-moderate renal or hepatic impairment. In patients with severe renal/hepatic impairment, rimonabant is not recommended, as further studies need to be conducted in those patient populations. Rimonabant has not been studied in patients being treated for epilepsy, necessitating caution in those patients. Since obesity is a condition that can be associated with depression, and depressive disorders were reported in rimonabant trials, rimonabant treatment should not be initiated in patients with uncontrolled serious psychiatric illnesses. Due to limited data in patients taking antidepressant medications, rimonabant is not recommended as concurrent therapy.6 More studies also need to be conducted in patients with cardiovascular events within 6 months of proposed rimonabant initiation, as these patients were excluded from rimonabant trials.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, clinical manager and director of Drug Information, Department of Pharmacy Services, Hartford Hospital, Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Richard D, Boisvert P. The endocannabinoid system and its role in energy homeostasis and abdominal obesity management. Int J Obes. 2006;30:s1–s2.
2. State-specific prevalence of obesity among adults-United States, 2005. MMWR. 2006;55:985–988. Available at: http:// http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5536a1.htm. Accessed October 30, 2006.
3. United States Department of Health and Human Services. The Surgeon General's call to action to prevent and decrease overweight and obesity. Available at: http:// http://www.surgeongeneral.gov/topics/obesity/calltoaction/1_3.htm. Accessed October 30, 2006.
4. Arterburn DE, Crane PK, Veenstra DL. The efficacy and safety of sibutramine for weight loss: a systematic review. Arch Intern Med. 2004;164:994–1003.
5. Padwal R, Li SK, Lau DCW. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes. 2003;27:1437–1446.
6. European Medicines Agency. Acomplia: European public assessment report. Available at: http:// http://www.emea.eu.int/humandocs/humans/epar/acomplia/acompliam.htm. Accessed October 30, 2006.
7. Sanofi-Aventis received from the FDA an approvable letter for rimonabant for weight management and a non approvable letter for smoking cessation [press release]. Paris, France: Sanofi-Aventis; February 17, 2006.
8. Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opin Investig Drugs. 2006;15:307–315.
9. Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview.Int J Obes. 2006;30(suppl 1):S13–S18.
10. Després J-P, Lemieux I, Alméras N. Contribution of CB1 blockade to the management of high-risk abdominal obesity. Int J Obes. 2006;30(suppl 1):S44–S52.
11. Turpault S, Kanamaluru V, Lockwood GF, Bonnet D, Newton J. Rimonabant pharmacokinetics in healthy and obese subjects [abstract]. In: Abstracts of the 2006 Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics (ASCPT); March 8–11, 2006; Baltimore, Md; USA. Clin Pharmacol Ther. 2006;79:50. Abstract PII-52.
12. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; for the RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397.
13. Després J-P, Golay A, Sjöström L; for the Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134.
14. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; for the RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775.
15. Finer N, Scheen AJ. Rimonabant improves blood glucose control and cardiometabolic profile in patients with type 2 diabetes: results of the RIO-diabetes study [abstract]. In: Abstracts of the Diabetes UK Annual Professional Conference, March 29–31, 2006; Birmingham, England, UK. Diab Med. 2006;23(suppl 2):11. Abstract A38.
16. Sanofi-Aventis. Clinical trial: Rimonabant In Obesity in Asia (RIO-Asia). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/NCT00325546?order=3. Accessed October 30, 2006.
17. Pi-Sunyer X, Després J-P, Scheen A, Van-Gaal L. Improvement of metabolic parameters with rimonabant beyond the effect attributable to weight loss alone: pooled 1-year data from the RIO (Rimonabant In Obesity and Related Metabolic Disorders) program. In: Abstracts of the 55th Annual Scientific Session of the American College of Cardiology (ACC); March 11–14, 2006; Atlanta, Ga, USA. J Am Coll Cardiol. 2006;47(suppl 1):362A. Abstract 849-3.
18. Niaura R. Long-term treatment with rimonabant for smoking cessation and the maintenance of abstinence: results from STRATUS-Worldwide trial. In: Abstracts of the 11th Annual Meeting and the 7th Annual European Conference of the Society for Research on Nicotine and Tobacco (SRNT), March 20–23, 2005; Prague, Czech Republic. Abstract POS1-054.
19. Anthenelli R, Després J-P. Effects of rimonabant in the reduction of major cardiovascular risk factors: Results from the STRATUS-US trial (Smoking Cessation in Smokers Motivated to Quit) and the RIO-LIPIDS trial (Weight Reducing and Metabolic Effects in Overweight/Obese Patients with Dyslipidemia). In: Abstracts of the 2004 Annual Scientific Session of the American College of Cardiology (ACC); March 7–10, 2004; New Orleans, La, USA. Abstract 409-1.
20. Gadde KM, Allison DB. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation.2006;114:974–984.
21. Scheen A, Finer N, Jensen M, Hollander P, Van Gaal L. Rimonabant improves cardiometabolic risk factors in overweight/obese patients with poorly controlled type 2 diabetes (HbA1c≥8%) on monotherapy with metformin or sulfonylureas [abstract]. Presented at: 42nd EASD Meeting 2006; September 14–17, 2006; Copenhagen, Denmark. Abstract 0798.
22. Sanofi-Aventis. Clinical trial: Study Evaluating Rimonabant Efficacy in Drug-NAive DiabEtic Patients. (SERENADE). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00257257?order=2.Accessed October 30, 2006.
23. Sanofi-Aventis. Clinical trial: ADAGIO-Lipids (An International Study of Rimonabant in Dyslipidemia With AtheroGenic Risk In Abdominally Obese Patients). In: ClinicalTrials.gov[Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/NCT00239967?order=2. Accessed October 30, 2006.
24. Sanofi-Aventis. Clinical trial: Study Evaluating Rimonabant Efficacy in Insulin-Treated Diabetic Patients (ARPEGGIO). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00288236?order=1. Accessed October 30, 2006.
25. Sanofi-Aventis. Clinical trial: Atherosclerosis Underlying Development Assessed by Intima-Media Thickness in Patients on Rimonabant (AUDITOR). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00228176?order=1. Accessed October 30, 2006.
26. Sanofi-Aventis. Clinical trial: STRADIVARIUS (Strategy To Reduce Atherosclerosis Development InVolving Administration of Rimonabant-the Intravascular Ultrasound Study). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/NCT00124332?order=4. Accessed October 30, 2006.
27. Sanofi-Aventis. Clinical trial: CRESCENDO Comprehensive Rimonabant Evaluation Study of Cardiovascular ENDpoints and Outcomes. In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00263042?order=1. Accessed October 30, 2006.
28. Sanofi-Aventis. Clinical trial: Rimonabant in Prediabetic Subjects To Delay Onset of Type 2 Diabetes (RAPSODI). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00325650?order=1. Accessed October 30, 2006.
29. National Institute on Alcohol Abuse and Alcoholism (NIAAA). Clinical trial: Rimonabant to Reduce Alcohol Consumption. In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00075205?order=1. Accessed October 30, 2006.
30. Sanofi-Aventis. Clinical trial: VICTORIA (VIsceral Fat Reduction Assessed by CT-scan On RImonabAnt). In: ClinicalTrials.gov [Internet]. Bethesda, Md: National Library of Medicine. Available at: http:// http://www.clinicaltrials.gov/ct/show/nct00299325?order=1. Accessed October 30, 2006.
