- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Rivaroxaban: a novel oral factor Xa inhibitor to prevent stroke in nonvalvular atrial fibrillation
Rivaroxaban is an oral, direct factor Xa inhibitor under review by FDA for stroke prevention in patients with atrial fibrillation. Atrial fibrillation conveys a 5-fold increased risk for stroke.

Key Points
Abstract
Rivaroxaban is an oral, direct factor Xa inhibitor under review by FDA for stroke prevention in patients with atrial fibrillation. Atrial fibrillation is the most common arrhythmia in the United States and conveys a 5-fold increased risk for stroke. Vitamin K antagonists, such as warfarin, remain the recommended antithrombotic treatment in this patient population, but clinical use of warfarin is limited by the need for frequent monitoring, prevalent and significant drug and food interactions, and genetic variability. As a result, alternatives that are more straightforward to manage are needed. It is likely that rivaroxaban will be administered in once-daily dose and will not require routine laboratory monitoring. Drug interactions have been reported but are limited to cytochrome P450 3A4 and P-glycoprotein. The efficacy and safety of warfarin and rivaroxaban were studied in a phase 3 trial. Preliminary findings established rivaroxaban as noninferior to warfarin. In addition, bleeding outcomes were similar between rivaroxaban and warfarin. (Formulary. 2011;46:257–267.)
Despite these recommendations, clinical concerns limit the use of warfarin. Once treatment with warfarin is initiated, patients require monitoring of their prothrombin time (PT) and international normalized ratio (INR) to confirm that both values are prolonged and thus the patient is anticoagulated. When above the narrow therapeutic window for INR, patients are at increased risk of bleeding complications whereas they are at greater thrombotic risk if below goal. Unfortunately, evidence suggests that patients are within the therapeutic range only about 50% of the time, which may account for a modest 35% decrease in stroke rates.5 Reasons for not maintaining the therapeutic range vary from case to case, but may be associated with the numerous food (particularly foods rich in vitamin K) and drug interactions (cytochrome P450 enzymes [CYP3A4, CYP2C9, CYP1A2], inducers and inhibitors) that antagonize anticoagulant effects or alter the metabolism of warfarin.4 Additional factors that complicate maintaining a therapeutic INR include genetic variability (CYP2C9 and VKORC1 polymorphisms) that affects warfarin metabolism as well as disease states (eg, congestive heart failure and thyroid disorders) that may affect bleeding risk.6 In addition, safety remains a concern. Several studies using usual care models (primary care provider) and specialized anticoagulation management services have demonstrated major hemorrhage rates from 1.4% to 8.1% in individuals taking warfarin for AF.4 Risks were highest in the elderly (>80 years) and those in whom more aggressive anticoagulation parameters were targeted.7
Given the concerns about warfarin, much effort has been dedicated to the search for a better agent for long-term oral anticoagulation. Recently, several new therapeutic agents have been evaluated, including an oral direct thrombin inhibitor, dabigatran (Pradaxa) that has received FDA approval for the prevention of stroke in patients with AF.8 On January 5, 2011, Johnson & Johnson Pharmaceutical Research & Development, LLC, submitted an NDA to FDA for a novel factor Xa inhibitor, rivaroxaban (Xarelto) for prevention of stroke in patients with AF.9 Like dabigatran, rivaroxaban is an oral agent that has the advantage of no required clinical monitoring, fixed dosing, and fewer interactions when compared with warfarin.8,10
CHEMISTRY AND PHARMACOLOGY
Rivaroxaban is an oxazolidinone derivative that binds directly and selectively to the active site of factor Xa. It inhibits factor Xa with more than 10,000-fold selectivity compared with other serine proteases.11
Rivaroxaban affects the clotting cascade by inhibiting both bound and free factor Xa. The primary effects are achieved by preventing the conversion of prothrombin to thrombin within the prothrombinase complex. In addition, rivaroxaban indirectly inhibits free factor Xa through an interaction with antithrombin. It has no antiplatelet aggregation activity and does not directly inhibit thrombin.10
Activity of rivaroxaban is dose dependent with the PT correlating with rivaroxaban concentrations.10 As a result, it may be possible to use PT for therapeutic monitoring if necessary.10 Routine laboratory monitoring is not recommended, however, and this is a proposed benefit over warfarin, which requires frequent monitoring.12
PHARMACOKINETICS
Upon oral administration, rivaroxaban is rapidly bioavailable at 80% with a time to peak concentration (Cmax) of 2 to 4 hours.10 Half-life is 7 to 11 hours in healthy volunteers. Single doses of rivaroxaban were associated with pharmacodynamic effects that persisted for 24 hours when administered to healthy subjects during phase 1 trials.13 In addition, rivaroxaban significantly inhibited peak and total amounts of thrombin generated and prolonged time to thrombin generation 24 hours after dosing in healthy subjects.13 As a result, once-daily dosing of rivaroxaban is being studied in phase 3 trials. Food does not affect rivaroxaban bioavailability. However, plasma protein binding with albumin is significant at approximately 92% to 95%.10
Two thirds of a dose of rivaroxaban is excreted via the renal route, and 28% via the fecal route.10 In patients with renal insufficiency (creatinine clearance [CrCl] <50 mL/min), additional exposure to rivaroxaban was seen with subsequent prolongation of PT and factor Xa inhibition as compared with control subjects.10 As a result, cautious use is warranted in patients with CrCl 30 to 50 mL/min. Rivaroxaban should not be used in patients with CrCl <30 mL/min.10 Rivaroxaban should also be avoided in patients with moderate-to-severe hepatic dysfunction (Child-Pugh class B or C) and in patients with liver disease associated with coagulopathy and clinically relevant bleeding risk.10
Rivaroxaban is metabolized via the CYP450 system, primarily through CYP3A4 and CYP2J2. The impact of polymorphisms of CYP450 on safety and efficacy has not been thoroughly studied. Clinically, known CYP3A4 inhibitors such as -azoles or inducers such as phenytoin will affect metabolism of rivaroxaban.10 Like dabigatran, rivaroxaban is also a substrate for transporter P-glycoprotein (P-gp) and, therefore, is affected by drugs that inhibit P-gp, such as verapamil, amiodarone, and clarithromycin, thereby potentiating anticoagulation.10
CLINICAL TRIALS
The potential application of rivaroxaban in multiple medical situations has been studied in clinical trials. Based on data from the Regulation of Coagulation in Major Orthopedic Surgery Reducing the Risk of DVT and PE (RECORD) 1-4 trials, the NDA for the indication of deep vein thrombosis (DVT) and pulmonary embolism (PE) prevention in major orthopedic surgery was submitted in July 2008.9,14–17 The FDA advisory committee found a favorable benefit versus risk profile for rivaroxaban in March 2009, and FDA granted approval for this indication on July 1, 2011.9,10
Rivaroxaban continues to be studied for additional indications. Rivaroxaban is in phase 2 trials for secondary prevention in acute coronary syndrome (Rivaroxaban versus Placebo in Patients with Acute Coronary Syndromes [ATLAS])18 and in phase 3 for the treatment of acute DVT (Einstein-DVT Dose-Ranging Study [EINSTEIN]), DVT prevention in the medically ill (Multicenter Randomized, Parallel Group Efficacy and Safety for the Prevention of VTE in Hospitalized Medically Ill Patients Comparing Rivaroxaban with Enoxaparin [MAGELLAN]), and for stroke and embolism prevention in AF (Rivaroxaban-Once daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in AF [ROCKET AF]).19–21
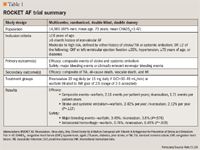
ROCKET AF was a randomized, double-blind, double-dummy, multicenter, event-driven trial that compared the efficacy of rivaroxaban and warfarin in patients with nonvalvular AF.21 With warfarin being the standard of care and showing high efficacy in previous trials, a noninferiority trial design was chosen; a placebo trial was deemed unethical. More than 14,000 patients were enrolled, and the study was completed in September 2010.21 Final results are yet to be released in a peer-reviewed journal. Preliminary findings were presented, however, at the American Heart Association 2010 Scientific Sessions.22,23
The study was conducted in 3 major phases-screening, double-blind randomization, and observation. After initial recruitment, patients completed a 14-day screening period before randomization. Participants were then randomly assigned to either rivaroxaban 20 mg daily (15 mg daily if CrCl <30-49 mL/min) or warfarin adjusted to a target INR of 2.5 (range, 2-3) for an expected total of 40 months (minimum 14 months and up to 4 years).23 Interventions such as concomitant antiarrhythmic drugs and aspirin therapy to 100 mg daily were allowed. However, concurrent use of thienopyridines within 5 days prior to randomization, fibrinolytic therapy within 10 days prior to randomization, nonsteroidal anti-inflammatory drug (NSAID) use for greater than 2 weeks, or certain CYP450 inhibitors were prohibited.23 Specific guidelines for stopping warfarin therapy 4 days prior and rivaroxaban 2 days prior to any procedure during the study were provided. Initial visits were on weeks 1 and 2, with follow up every 4 weeks. The final visit occurred within 30 days of finishing the study, when patients were transitioned to another appropriate preventative therapy.21
The study was conducted at 1,100 sites in 45 different countries.23 Inclusion criteria included age ≥18 years, a minimum 6-month history of nonvalvular AF, and additional risk factors, such as history of stroke, transient ischemic attack (TIA), systemic embolism, or 2 or more of the following CHADS2 risk factors: clinical heart failure, left ventricular ejection fraction ≤35%, hypertension, ≥75 years of age, or diabetes mellitus. The CHADS2 index is used by the ACCP to stratify risk of stroke in nonvalvular AF patients.4 At baseline, participants had multiple comorbidities, approximately 90% having a CHADS2 score ≥3, and would thereby be categorized as high risk for stroke, warranting warfarin therapy.23 Special considerations were made to enroll patients who were naïve to both study medications. Patients were excluded if they had a prosthetic heart valve, active infection, mitral stenosis that caused hemodynamic instability, planned cardioversion, secondary AF, active bleeding or history of increased bleeding risk, stroke during screening period, CrCL <30 mL/min, or TIA within 3 days of randomization, or anticoagulant therapy for conditions other than AF.21
The primary study outcome was a composite of ischemic or hemorrhagic stroke and systemic embolism. Secondary efficacy end points included events such as TIA, all-cause death, vascular death, and myocardial infarction. The primary safety end point was a composite of major and clinically relevant nonmajor bleeding events. An event was considered "major bleeding" if there was excess bleeding with either a fatal outcome, decrease in hemoglobin concentration ≥2 g/dL, involvement of a critical site (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal), or need for ≥2 units of packed red blood cells or whole blood.21 "Nonmajor bleeding" events were defined as excess bleeding not meeting the above criteria, but either impairing daily activities, causing pain, requiring a physician visit outside of scheduled visits, occurring as a result of a medical intervention, or causing temporary suspension of study drug. All other bleeding events were considered minor.21
The study was event driven and powered to determine noninferiority to warfarin in preventing the primary end points. As a result, the study was continued until 405 events were observed. If noninferiority to warfarin had been demonstrated with primary outcomes, further analysis was planned to determine possible superiority of rivaroxaban with respect to primary and secondary efficacy end points and primary safety end points.21
The mean age of the study population was 73 years and 60% were men; 55% had experienced previous stroke/TIA.22 Approximately 37.5% were warfarin-naïve, and the mean CHADS2 score was 3.47.22,23 Median follow-up time was 19 months. By study conclusion, rivaroxaban noninferiority to warfarin was demonstrated in the primary end point with 1.71 events per patient-years in the rivaroxaban group compared with 2.16 in the warfarin group (P<.001).23 Intent-to-treat analysis did not demonstrate rivaroxaban superiority, with 2.42% of warfarin and 2.12% of rivaroxaban patients having a stroke or systemic embolism per year (P=.117). In a pre-specified subgroup analysis of the 7,468 patients with a prior history of stroke or TIA, rivaroxaban-treated patients had a 13% lower risk of recurrent stroke or systemic embolism as compared to warfarin-treated patients, though these results are not statistically significant and the study was not powered to detect these differences.24
Major and nonmajor bleeding events remained similar between the 2 groups, with 14.91 events occurring in the rivaroxaban group and 14.52 in the warfarin group (P=.442). A statistically significant difference was seen in rates of intracranial hemorrhage, 0.49% in the rivaroxaban group and 0.74% in warfarin (P=.019).22 The investigators concluded that the more stringent intent-to-treat analysis revealed rivaroxaban was not superior, but was noninferior to warfarin in this moderate-to-high risk population.23 In addition, bleeding outcomes were similar in both groups.
ADVERSE EVENTS
Rivaroxaban inhibits the activity of factor Xa and by design inhibits clot formation. In light of this pharmacologic mechanism, it would be expected to be associated with some increase in major and minor bleeding events. It will be important to characterize this risk relative to current standard anticoagulant therapies. In addition, because of risk of hepatotoxicity associated with the oral, direct thrombin inhibitor ximelagatran, pre- and postapproval surveillance should specifically assess hepatic enzymes and other indicators of liver function.25
Adverse events in the RECORD study program have been reviewed in this journal in a previous article.26 Since that publication, a meta-analysis of randomized controlled trials evaluating rivaroxaban for VTE prophylaxis after orthopedic surgery similarly found no significant difference in bleeding events between rivaroxaban- and exonaparin-treated groups.27 Adverse event data from the EINSTEIN-Acute DVT study also revealed no significant difference in adverse events between rivaroxaban- and enoxaparin-treated patients in rates of major bleeding or total deaths, and no difference was seen between rivaroxaban or enoxaparin-treated groups with respect to evidence of liver toxicity during the treatment period.28 In the continued treatment study, more rivaroxaban-treated patients experienced increases in alanine aminotransferase (ALT) greater than 3 times the upper limit of normal (11 vs 3 with rivaroxaban and placebo, respectively) or 5 times the upper limit of normal (2 vs 0 with rivaroxaban and placebo, respectively).28
The results of the ROCKET AF trial have not been reported in full. The abstracted report of study results describes similar rates of major or minor bleeding in rivaroxaban- and warfarin-treated patients (14.91 vs 14.52 per 100 patient-years for rivaroxaban and warfarin groups, respectively, P=NS). Rivaroxaban-treated patients experienced fewer intracranial hemorrhages and fatal bleeding events than those treated with warfarin. Other safety data were not reported.23,29
The FDA-approved prescribing information also includes a boxed warning about the risk of spinal/epidural hematoma in patients who are anticoagulated and receiving spinal procedures.10
DRUG INTERACTIONS
Rivaroxaban is a substrate of CYP450 3A4 and P-gp and may interact with other agents that induce and inhibit these mechanisms.10 The FDA-approved prescribing information for rivaroxaban recommends that patients receiving rivaroxaban not receive concomitant treatment with agents that inhibit both CYP3A4 and P-gp.10 If a patient needs concomitant treatment with a combined P-gp and CYP3A4 inducer, clinicians should consider alternatives or an increased dose of rivaroxaban.10
The effects of concomitant administration of the NSAIDs naproxen and aspirin have also been evaluated.30,31 In both of these studies, coadministration did not significantly affect platelet aggregation or the antithrombotic effect of rivaroxaban, but did increase bleeding time when compared with either drug alone. In each case, the investigators concluded these increases did not constitute a clinically important interaction. Concomitant use with clopidogrel resulted in no additional effect on platelet aggregation or factor Xa activity, but an increase in bleeding time was observed in a few subjects, similar to the studies of aspirin and naproxen. The authors suggest this effect may not be of clinical concern, although they concluded that coadministration warranted additional study.32 The US prescribing information recommends that concurrent use be avoided unless expected benefit outweighs risk.10
The effects of food, antacids, and ranitidine on rivaroxaban have also been evaluated.33 Administration of rivaroxaban with food resulted in a moderate increase in oral absorption, Cmax, and time to Cmax by 1.5 hours. The elimination half-life remained unchanged in the presence of food. Coadministration with antacids or ranitidine did not affect rivaroxaban. The US prescribing information states that the drug may be taken with or without food.10 Likewise, no clinically important interaction has been found between rivaroxaban and digoxin or atorvastatin, and available evidence indicates that rivaroxaban may be used together with these agents.34,35
DOSING AND ADMINISTRATION
Rivaroxaban is not yet FDA approved for the prevention of thromboembolic stroke in patients with AF, so dosing recommendations are not yet available. For the prevention of DVT in patients undergoing orthopedic surgery, the usual adult dose is 10 mg by mouth once daily.10 In the ROCKET AF study, rivaroxaban was administered as 20 mg once daily. According to investigators, this dose was chosen out of a range of previously studied doses because of evidence of efficacy, but a lower risk of bleeding, taking into account the likely age and comorbidities of the patients with AF who would ultimately use rivaroxaban.21
Adjustments in rivaroxaban dose are not currently required for body weight, gender, age, or ethnicity.10,36
Kubitza and associates found that renal insufficiency does affect clearance of rivaroxaban and results in increased exposure to the drug.37 In this study, however, the elimination half-life increased only slightly, even in patients with severe renal impairment. FDA-approved labeling recommends that rivaroxaban be used with caution in those with CrCl 30 to 50 mL/min and that it not be used in those with CrCl <30 mL/min.10 Patients with CrCl <30 mL/min at the screening visit were excluded from the ROCKET AF trial.21 Rivaroxaban is also not recommended for patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk or those with moderate-to-severe hepatic impairment (Child-Pugh class B or C).10 Patients with significant liver disease or ALT >3 times the upper limit of normal were excluded from the ROCKET AF trial.21
FORMULARY CONSIDERATIONS
Rivaroxaban is under investigation for the prevention of stroke in patients with nonvalvular AF. It was FDA approved July 1, 2011 for the DVT prevention indication and will be marketed under the trade name Xarelto. The standard of care for stroke prevention in patients with AF and at intermediate to high risk (CHADS2 score ≥1) is warfarin (adjusted to target INR of 2-3). If approved, rivaroxaban would be positioned for use as an alternative to warfarin as it requires no routine laboratory monitoring and has fewer drug interactions and no significant dietary restrictions. It also appears not to be associated with significant intra- or interpatient variability.10
Review of this agent for the indication of stroke prevention in AF is limited because it has been studied in only 1 large-scale, noninferiority trial, and results have been released only in abstract format for presentation at the AHA 2010 Scientific Sessions.22,23 ROCKET AF, a phase 3 trial, demonstrated rivaroxaban noninferiority to warfarin in preventing the primary end point of stroke and noncentral nervous system embolism. However, superiority to the standard of care, warfarin, was not demonstrated. In addition, safety end points between rivaroxaban and warfarin were similar. Based on these preliminary reports, rivaroxaban seems to have similar efficacy to warfarin in stroke prevention and may be a reliable alternative to warfarin.
Rivaroxaban may have an advantage in terms of safety considerations. In the ROCKET AF trial, rates of major bleeding were not significantly different between rivaroxaban-treated and warfarin-treated patients, but rates of intracranial hemorrhage, bleeding mortality, and critical organ bleeding were statistically significantly lower in the rivaroxaban group. From other phase 2 and 3 studies, rivaroxaban's adverse event profile seems limited to transient increases in ALT, as previously mentioned.28 Long-term safety data are currently unavailable from clinical trials of rivaroxaban.
Rivaroxaban compares favorably to other recommended agents for stroke prevention based on its oral dosage formulation, once-daily administration, and standard, consistent daily dosing regimen. It would likely not require routine laboratory monitoring or dose adjustment. In cases where anticoagulation monitoring is desirable, it may be possible to monitor PT or factor Xa activity. One notable disadvantage with rivaroxaban is its lack of reversibility. In an overdose or emergent situation, there are no currently available antidotes that would rapidly reverse anticoagulation.
Although rivaroxaban does not require dose adjustment based on age, gender, or weight, it is renally eliminated and is not recommended for use in patients with CrCl <30 mL/min or in those on hemodialysis. It also is not appropriate for treatment of stroke or in cases where rapid anticoagulation reversibility is desired.
According to the manufacturer, the US cost is $6.75 per 10-mg tablet of Xarelto.38 Although the dose for stroke prevention is not yet established, further cost-effectiveness analysis would be needed to compare warfarin and rivaroxaban. Based on available information, rivaroxaban seems to be a promising agent for stroke prevention and an interesting alternative to warfarin. Its first applications may likely be in patients with difficult-to-control INRs or those unable to adhere to laboratory monitoring guidelines for warfarin. Additional clinical studies will need to be conducted and evaluated before an informed decision can be made. It will be important to consider small differences in adverse event rates carefully in light of similar efficacy data. Furthermore, without superiority data for rivaroxaban, patients at high risk for stroke and adherent to warfarin monitoring parameters may not be the preferred candidates until additional data become available.
Rivaroxaban will be compared to dabigatran, an oral, direct thrombin inhibitor recently FDA approved for stroke prevention in this patient population. Dabigatran has no known drug interactions via the CYP450 system, but similar to rivaroxaban, is affected by the P-gp system.8 One difference between the 2 new agents is the proposed once-daily dosing of rivaroxaban compared with twice-daily dosing with dabigatran. Dabigatran was evaluated in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial, which enrolled 18,113 patients randomly assigned to treatment with dabigatran 150 mg twice daily or warfarin in a noninferiority design.39 Of particular importance to note, at baseline, patients differed in the dabigatran and rivaroxaban studies, with higher risk patients in the rivaroxaban study. In the RE-LY trial, average CHADS2 score was 2.1, corresponding with an intermediate risk of stroke whereas in ROCKET AF, the average CHADS2 score was 3.47, corresponding with a high risk of stroke.23,39 In RE-LY, the investigators found dabigatran 150 mg twice daily to be superior to warfarin, with a relative risk of 0.66 (P<.001).39 No head-to-head trials of dabigatran versus rivaroxaban for stroke prevention have been conducted.
Rivaroxaban is an interesting potential addition to available anticoagulants. Given the well-established place of warfarin in stroke prevention, any new agent must be carefully and thoroughly evaluated, but rivaroxaban offers important potential advantages to providers, payers, and patients.
Dr Vitin is an assistant clinical professor at Northeastern University in Boston, and clinical pharmacist at Lynn Community Health Center in Lynn, Mass. Dr Quinto is a pharmacy practice resident at Grandview Medical Center in Dayton, Ohio. Dr Kirwin is an associate clinical professor at Northeastern University in Boston.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, medical editor, University of Connecticut/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn., and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123; e18-209.
2. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98;946-952.
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22;983-988.
4. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists. American College of Chest 5. 5 5. 5. Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133; 160S-198S.
5. Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009; 15(3):244-252.
6. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2010.
7. Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med.1994;120(11);897-902.
8. Pradaxa [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2011.
9. Johnson & Johnson. Major search engines and directories. Rivaroxaban virtual media kit. http://www.jnjpharmarnd.com/jnjpharmarnd/rivaroxaban.html. Updated April 5, 2011. Accessed May 11, 2011.
10. Xarelto [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
11. Roehrig S, Straub A, Pohlmann J, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5 yl} methyl) thiophene-2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem. 2005;48:5900-5908.
12. Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban-an oral, direct factor Xa inhibitor-in healthy subjects. Int J Clin Pharmacol Ther. 2007;45(6):335-344.
13. Harder S, Graff J, Hentig NV, et al. Effects of BAY 59-7939, an oral, direct Factor Xa inhibitor, on thrombin generation in healthy volunteers. Pathophysiol Haemos. 2004;33(suppl 2):97. Abstract PO078.
14. Eriksson BI, Borris LC, Friedman RJ, et al; for the RECORD 1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765-2775.
15. Lassen MR, Ageno W, Borris LC, et al; for the RECORD 3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776-2786.
16. Kakkar AK, Brenner B, Dahl OE, et al; for the RECORD 2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled study. Lancet. 2008;372(9632);31-39.
17. Turpie AG, Lassen MR, Davidson BL, et al; for the RECORD 4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009; 373;1673-1680.
18. Mega JL, Braunwald E, Mohanavelu S, et al; for the ATLAS ACS-TIMI 46 Study Group. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009; 374(9683):29-38.
19. Buller HR, Lensing AWA, Prins MH, et al; for the Einstein-DVT Dose-ranging Study Investigators. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-ranging Study. Blood. 2008;112(6):2242-2247.
20. Cohen AT, Spiro TE, Büller HR, et al; for the MAGELLAN Steering Committee. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011;DOI 10.1007/s11239-011-0549-x.
21. The ROCKET AF Investigators. Rivaroxaban-Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in AF: Rationale and Design of the ROCKET AF study. Am Heart J. 2010;159:340-347.
22. Ahrens I, Lip GYH, Peter K. What do the RE-LY, AVERROES and ROCKET-AF trials tell us for stroke prevention in AF? Thromb Haemost. 2011;105;574-578.
23. Cleland JGF, Coletta AP, Buga L, et al. Clinical trials update from the American Heart Association Meeting 2010: EMPHASIS-HF, RAFT, TIM-HF, Tele-HF, ASCEND-HF, ROCKET-AF, and PROTECT. Eur J Heart Fail. 2011;13:460-465.
24. Keller DM. Rivaroxaban effective for secondary stroke prevention in AF. May 27, 2011. http://Medscape.com/. Available at http://www.medscape.com/viewarticle/743544. Accessed June 20, 2011.
25. Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol. 2010;196:407-418.
26. Nunokawa N, Wong H, Song JC. Rivaroxaban: A direct factor Xa inhibitor for VTE prophylaxis in patients undergoing total knee or hip replacement surgery. Formulary. 2009;44:226-236. Available at: http://formularyjournal.modernmedicine.com/formulary/Modern+Medicine+Now/Rivaroxaban-A-direct-factor-Xa-inhibitor-for-VTE-p/ArticleStandard/Article/detail/616632. Accessed June 6, 2011.
27. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2010;66:1099-1108.
28. EINSTEIN investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510.
29. O'Riordan M. Off orbit? ROCKET AF: Rivaroxaban noninferior to warfarin, but superiority analyses at odds. Available at: http://www.theheart.org/article/1148785.do. Accessed April 26, 2011.
30. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Rivaroxaban (BAY 59-7939)-an oral direct factor Xa inhibitor-has no clinically relevant interaction with naproxen. Br J Clin Pharmacol. 2006;63(4):469-476.
31. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Safety, tolerability, pharmacodynamics and pharmacokinetics or rivaroxaban-an oral direct factor Xa inhibitor-are not affected by aspirin. J Clin Pharmacol. 2006;46:981-990.
32. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Co-administration of rivaroxaban-a novel, oral, direct factor Xa inhibitor-and clopidogrel in healthy subjects [abstract]. Eur Heart J. 2007;28(suppl 1):Abstract 189.
33. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46:549-558.
34. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. No interaction between the novel, oral direct factor Xa inhibitor BAY 59-7939 and digoxin. [abstract]. J Clin Pharmacol. 2006;46:702. Abstract 11.
35. Kubitza D, Mueck W, Becka M. No interaction between rivaroxaban-a novel, oral, direct factor Xa inhibitor-and atorvastatin [poster]. Presented at: the 20th International Congress on Thrombosis (ICT); June 25–28, 2008; Athens, Greece. Poster P062.
36. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47:218-226.
37. Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacology. 2010;70(5):703-712.
38. FDA approves new anticlot drug from J&J and Bayer. Fox Business. July 1, 2011. Available at: http://www.foxbusiness.com/markets/2011/07/01/fda-approves-new-anticlot-drug-from-jj-and-bayer/. Accessed July 7, 2011.
39. Connolly SJ, Ezekowitz MB, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361(12);1139-1151.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
