
- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Rosiglitazone associated with increased risk of MI and cardiovascular-related death
Rosiglitazone is associated with an increased risk of myocardial infarction [MI] and death from cardiovascular causes, according to findings of a meta analysis.

Key Points

The meta-analysis included pooled results of 42 randomized, controlled trials involving 27,847 patients. Patients received either rosiglitazone (n=15,565) or placebo or active comparators (n=12,282). All data used for this analysis were available in published literature, on FDA's website, or in a registry of clinical trials maintained by GlaxoSmithKline. All included trials had a mean duration of patient follow-up of ≥24 weeks and reported data on the incidence of MI or death from cardiovascular causes.
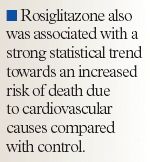
The authors offered several plausible explanations for the increased cardiovascular risk associated with rosiglitazone, including the drug's undesirable effect on low-density lipoprotein (LDL) cholesterol (an effect not demonstrated with pioglitazone), the drug's propensity to cause congestive heart failure, and the modestly reduced hemoglobin levels often associated with use of rosiglitazone.
According to the American Diabetes Association, 20.8 million children and adults in the United States, or 7% of the population, have diabetes. Among these patients, type 2 diabetes accounts for approximately 90% to 95% of all diagnosed cases. Heart disease and stroke account for approximately 65% of deaths in patients with diabetes. The risk of cardiovascular-related death is 2 to 4 times greater and the risk of death from stroke is 2.8 times greater among adults with diabetes compared with adults without diabetes.
According to the authors: "Because exposure of [patients with diabetes] to rosiglitazone is widespread, the public health impact of an increase in cardiovascular risk could be substantial if our data are borne out by further analysis and the results of larger controlled trials."
"Until more precise estimates of the cardiovascular risk of this treatment can be delineated in patients with diabetes, patients and providers should carefully consider the potential risks of rosiglitazone in the treatment of type 2 diabetes," the authors stated.
The Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) trial is currently under way and may provide the answers to questions concerning rosiglitazone's cardiovascular safety.
EDITORS' NOTES: Since the publication of this meta-analysis in the NEJM, FDA has informed healthcare professionals of the potential safety issues related to rosiglitazone use. FDA stated that, based on the data from this and other studies, the potential risk of ischemic cardiovascular events due to rosiglitazone remains unclear. Prescribers were advised to continue making careful, individualized treatment decisions for patients with diabetes mellitus.
In addition, FDA Commissioner Andrew von Eschenbach, MD, said at a congressional hearing on June 6, 2007, that FDA will strengthen the cardiovascular warnings on the labels of rosiglitazone and pioglitazone.
An FDA advisory panel is scheduled to convene on July 30, 2007, to discuss the cardiovascular risks associated with rosiglitazone.
SOURCES
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes [correction is available at http:// http://content.nejm.org/cgi/content/full/357/1/100. Accessed June 14, 2007]. N Engl J Med. 2007;356:2457–2471.
Complications of diabetes in the United States. American Diabetes Association website. http:// http://www.diabetes.org/diabetes-statistics/complications.jsp. Accessed June 14,2007.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
2 Commerce Drive
Cranbury, NJ 08512