- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Sitagliptin: The first dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes
A variety of clinical approaches are utilized in the management of poor glycemic control in patients with type 2 diabetes. Sitagliptin (Januvia, Merck), a novel drug in a new medication class known as dipeptidyl peptidase-IV (DPP-IV) inhibitors, offers a new mechanism by which to achieve glycemic control. Although stimulation of receptors by the glucagon-like peptide-1 (GLP-1) enhances the body's ability to produce insulin in response to elevated blood glucose concentrations, rapid degradation of GLP-1 by DPP-IV limits its clinical effectiveness. The development of medications to reduce this degradation is being pursued by numerous manufacturers. An NDA for the first of these medications, sitagliptin, was submitted to FDA in February 2006. Currently available clinical studies have demonstrated improved glycemic control with sitagliptin therapy in patients who have not achieved target glucose levels with diet and oral medications. (Formulary. 2006;41:434–441.)
Abstract
A variety of clinical approaches are utilized in the management of poor glycemic control in patients with type 2 diabetes. Sitagliptin (Januvia, Merck), a novel drug in a new medication class known as dipeptidyl peptidase-IV (DPP-IV) inhibitors, offers a new mechanism by which to achieve glycemic control. Although stimulation of receptors by the glucagon-like peptide-1 (GLP-1) enhances the body's ability to produce insulin in response to elevated blood glucose concentrations, rapid degradation of GLP-1 by DPP-IV limits its clinical effectiveness. The development of medications to reduce this degradation is being pursued by numerous manufacturers. An NDA for the first of these medications, sitagliptin, was submitted to FDA in February 2006. Currently available clinical studies have demonstrated improved glycemic control with sitagliptin therapy in patients who have not achieved target glucose levels with diet and oral medications. (Formulary. 2006;41:434–441.)
An estimated 194 million people worldwide have diabetes, accounting for more than 5% of the adult population.1 In the United States alone, an estimated 20.8 million people have diabetes.2 The prevalence of diabetes is predicted to increase exponentially over the next 2 decades, with 333 million adults diagnosed worldwide by 2025.1 Early reports from the 2005 National Health Interview survey indicate an increase in the prevalence of diagnosed diabetes in US adults aged >18 years from 5.1% in 1997 to 7.4% in 2005.3 This prevalence increases with age, with 17% of adults aged ≥65 years reporting a diagnosis of diabetes.3

Despite the benefits of lower HbA1c values, studies show that many patients are not achieving these goals.5 The American Diabetes Association (ADA) recommends an HbA1c goal for patients in general of <7%, with the caveat that the ideal goal for the individual patient is an HbA1c as close to normal (<6%) as possible without significant hypoglycemia.6 In an attempt to improve glycemic control by offering new oral agents, numerous pharmaceutical companies are studying compounds that affect incretin hormones. Incretin hormones stimulate insulin release in response to increased glucose levels. Sitagliptin (Januvia, Merck) is the first agent in a new class of medications known as dipeptidyl peptidase IV (DPP-IV) inhibitors. This once-daily medication is initially being investigated to improve glycemic control in patients with type 2 diabetes who are not taking insulin and are not achieving target glucose levels with lifestyle modifications and current oral medications. An NDA for sitagliptin was submitted in February 2006. At the time of publication, a response was expected from FDA by mid-October 2006.
CHEMISTRY AND PHARMACOLOGY
Sitagliptin is an orally active, potent, and selective inhibitor of DPP-IV. DPP-IV inhibitors enhance levels of active GLP-1, facilitating glucose-dependent insulin secretion.
GLP-1 is an enteric hormone secreted in the small intestine in response to nutrient ingestion. Interest in GLP-1 is based on its ability to amplify glucose-stimulated insulin secretion. In contrast to agents that promote insulin secretion via glucose-independent mechanisms, GLP-1's dependence on glucose concentration is considered a unique safety advantage due to lower risk of hypoglycemia. However, the utility of GLP-1 is limited by its short half-life (2 minutes) secondary to rapid degradation by the proteolytic enzyme DPP-IV. DPP-IV exerts its biological effects via 2 mechanisms. First, DPP-IV's enzymatic activity is exhibited through the membrane-spanning form of the molecule and the circulating soluble form. DPP-IV preferentially cleaves substrates with an amino-terminal proline or alanine at position, including GLP-1. Second, DPP-IV, independent of its enzymatic properties, binds adenosine deaminase and conveys intracellular signals via dimerization and activation of intracellular pathways. The signaling properties of DPP-IV are characterized on T cells, leading to a functional role in T cell activation and proliferation.
The prevention of GLP-1 degradation through the inhibition of the DPP-IV enzyme is emerging as a therapeutic strategy to enhance GLP-1 activity. The administration of sitagliptin enhances GLP-1's ability to produce insulin in response to elevated concentrations of blood glucose, to inhibit the release of glucagon following meals, to slow the rate of nutrient absorption into the bloodstream, to slow the rate of gastric emptying, and to reduce food intake.
PHARMACOKINETICS
The pharmacokinetic profile of sitagliptin in healthy human subjects was described after single oral dosing. Median Tmax values ranged from 1 to 6 hours. A high-fat breakfast did not have a clinically significant effect on the extent or rate of absorption. Sitagliptin is primarily cleared via the kidney, demonstrating a half-life of approximately 8 to 14 hours. Renal clearance was independent of dose, averaging 388 mL/min. The fraction of sitagliptin excreted unchanged in urine is approximately 80%.7 Six metabolites have been identified in the plasma, urine, and feces of patients. The fecal route accounts for 13% of elimination.8
In multiple dose studies, sitagliptin trough concentrations indicated steady-state plasma concentrations were achieved by day 2. Sitagliptin plasma AUC increased dose-proportionally over 10 days of therapy. Cmax increased in a slightly greater than dose-proportional manner.9 Sitagliptin exposure is increased in patients with impaired renal function.10 The increase is approximately 2.3-fold relative to healthy subjects for patients with moderate renal impairment (creatinine clearance, 30–49 mL/min), 3.8-fold with severe renal impairment (creatinine clearance <30 mL/min), and 4.5-fold for patients on dialysis.
In a study conducted to evaluate the influence of hepatic insufficiency on sitagliptin pharmacokinetics, moderate hepatic insufficiency had no statistically significant effect on Tmax, half-life, renal clearance, or fraction of oral dose excreted in urine.11
CLINICAL TRIALS
In a double-blind, placebo-controlled, 3-period, single-dose, crossover study, researchers evaluated 56 patients with type 2 diabetes.12 After an overnight fast, patients were given sitagliptin 25 mg or 200 mg or placebo, with a 1-week washout between doses. Oral glucose tolerance testing performed 2 hours after dosing showed sitagliptin significantly reduced the incremental AUC for glucose by 22% and 26%, respectively (P<0.001). Both sitagliptin doses exhibited 2-fold increases in GLP-1 concentrations, increases in plasma insulin AUC, and increases in C-peptide AUC. The plasma glucagon AUC decreased by 8% to 14%. No immunologic effects were observed.
Sitagliptin increased nutrient-stimulated active GLP-1 levels approximately 2-fold in a study involving healthy male volunteers. Twenty-four hours after administration, DPP-IV inhibition was ≥80% for doses greater than 100 mg daily.13 Approximately 50% of DPP-IV inhibition occurred at a sitagliptin plasma concentration of 26 nmol/L and 80% inhibition at 100 nmol/L.14
Nonaka et al15 performed a 12-week randomized, double-blind, placebo-controlled, parallel-group study to determine sitagliptin's ability to improve HbA1c. At Week 12, HbA1c decreased by 0.65% from a mean baseline of 7.5% in the sitagliptin-treated patients. Patients who were administered placebo demonstrated an HbA1c increase of 0.41% from a mean baseline of 7.7%. The between-treatment group difference in HbA1c was –1.05% (P<.001). In the sitagliptin group, 58.1% of patients achieved an HbA1c <7%. Only 14.5% achieved this goal in the placebo group.
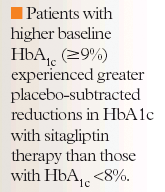

Researchers also evaluated the use of sitagliptin in combination with currently available antihyperglycemic agents. Mu and colleagues examined the effects of des-fluoro-sitagliptin, an analog of sitagliptin, on glycemic control and pancreatic islet function in mice.18 The effects of des-fluoro-sitagliptin were compared with those of rosiglitazone and glipizide. Following 10 weeks of therapy, des-fluoro-sitagliptin-treated mice demonstrated significantly improved postprandial and fasting hyperglycemia, HbA1c, circulating insulin, triglycerides, and free fatty acid levels. These mice also demonstrated significantly increased beta cell mass, improved glucose-stimulated insulin secretion, and reduced circulating and pancreatic glucagon levels. Rosiglitazone-treated patients demonstrated comparable improvement in plasma glucose and islet function, but less favorable effects on glucose tolerance and liver triglycerides. This may be due to body weight gain and fat accumulation due to chronic rosiglitazone therapy. Glipizide-treated patients demonstrated plasma glucose control during the first weeks of therapy, but glipizide efficacy declined over time, possibly due to beta cell exhaustion.

In a larger study, Karasik et al21 assessed the addition of sitagliptin 100 mg/d for 24 weeks in 701 patients with inadequate glycemic control (HbA1c from 7% to 10%) on metformin >1,500 mg/d. After 24 weeks, the addition of sitagliptin led to a placebo-subtracted reduction in HbA1c (–0.65%), fasting plasma glucose (–25.4 mg/dL), and 2-hour post-meal plasma glucose (–50.6 mg/dL) (P<.001 for all 3 measures). Relative to placebo, fasting and post-meal insulin, fasting and post-meal C-peptide AUC, and post-meal insulin AUC/glucose AUC ratio were all significantly increased with sitagliptin therapy. Sitagliptin-treated patients also demonstrated increased HOMA-B and decreased proinsulin/insulin ratio (both indicators of improved beta cell function). Mean body weight change was not different between sitagliptin and placebo-treated patients.

ADVERSE EVENTS
In studies, sitagliptin was generally well tolerated. Reported adverse events were mild-to-moderate with the most common being headache, diarrhea, joint pain, and nausea and vomiting (9%).22 Sitagliptin was not associated with weight gain from baseline. In single-dose studies, sitagliptin was not associated with hypoglycemia with doses up to 600 mg.23 In 28-day, multiple dose studies, no hypoglycemia was experienced.24
One study found a similar overall incidence of adverse effects when patients with renal impairment were treated with dose-adjusted sitagliptin.25 Patients with moderate renal impairment received 50 mg daily, while patients with severe or end-stage renal disease received 25 mg daily. The incidences of medication-related adverse events, serious adverse events, and discontinuation due to adverse events were modestly higher with sitagliptin than placebo. No particular adverse event exhibited a significantly higher incidence. No differences were noted in the incidence of hypoglycemia or gastrointestinal adverse events.
DRUG INTERACTIONS
Currently there is no published information documenting medications that interact with sitagliptin. This may be related to the fact that it does not appear to be cleared through the CYP450 enzyme system. In studies, no statistically or clinically significant pharmacokinetic interactions occurred with warfarin,26 glyburide,27 metformin,28 rosiglitazone,29 cyclosporine,30 or simvastatin.31
DOSING AND ADMINISTRATION
Sitagliptin was still undergoing FDA review at the time of publication. However, based on clinical trials, oral sitagliptin 25 to 400 mg once daily produced long-lasting DPP-IV inhibition over a 24-hr period,32 with 100 mg once daily being the most common dosing regimen utilized in study protocols. Due to its renal elimination, the sitagliptin dose should be adjusted in patients with impaired renal function.
Dr Schlesselman is assistant clinical professor of pharmacy practice,University of Connecticut School of Pharmacy, Storrs, Conn. She can be reached at lauren.schlesselman@uconn.edu
Disclosure Information: The author reports no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital in Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. International Diabetes Federation. Diabetes Atlas: Executive Summary. 2nd edition. Available at: http:// http://www.eatlas.idf.org/webdata/docs/Atlas%202003-Summary.pdf. Accessed August 21, 2006.
2. Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2005. Available at: http:// http://www.diabetes.org/uedocuments/NationalDiabetesFactSheetRev.pdf. Accessed August 21, 2006.
3. Centers for Disease Control and Prevention. NCHS-NHIS-Data from the January-September 2005 (Released 03/2006). Available at: http:// http://www.cdc.gov/nchs/about/majornhis/released200603.htm. Centers for Disease Control and Prevention website. Accessed August 21, 2006.
4. Hogan P, Dall T, Nikolov P; American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932.
5. Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–408.
6. American Diabetes Association. Standards of medical care in diabetes-2006. Diabetes Care. 2006;29(suppl 1):S4–S42.
7. Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharm Ther. 2005;78:675–688.
8. Vincent S, Reed JR, Bergman A, et al. Metabolism and excretion of the DPP-IV inhibitor [14 C]sitagliptin (MK-0431) in humans [abstract]. J Clin Pharmacol. 2005;45:1067–1096. Abstract 87.
9. Narita H, Nonaka K, Stevens C, Okuyama K, Fumimoto G, et al. Multiple dose administration of sitagliptin, a dipeptidyl peptide IV (DPP-IV) inhibitor, in healthy Japanese subjects [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 6–13, 2006; Washington, DC. Abstract 533-P.
10. Scott RS, Hartley P, Luo E, et al. Use of sitagliptin in patients with type 2 diabetes (T2DM) and renal insufficiency (RI) [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 6–13, 2006; Washington, DC. Abstract 1997-PO.
11. Stevens C, Bergman AJ, Liu Q, Luo W, Wang AQ, et al. Lack of clinically significant effect of moderate hepatic insufficiency on the pharmacokinetics of MK-0431 (sitagliptin), a dipeptidyl-peptidase-IV (DPP-IV) inhibitor [abstract]. Clin Pharm Ther. 2006;79;P49. Abstract PII-49.
12. Herman G, Zhao PL, Dietrich B, et al. The DP-IV inhibitor MK-0431 enhances active GLP-1 and reduces glucose following an OGTT in type 2 diabetics [abstract]. Diabetologia. 2004;47(suppl 1):A287.
13. Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72.
14. Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharm Ther. 2005;78:675–688.
15. Nonaka K, Kakikawa T, Sato A, et al. Twelve-week efficacy and tolerability of sitagliptin, a dipeptidyl peptidase-IV (DPP-4) inhibitor, in Japanese patients with T2DM [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13 2006; Washington, DC. Abstract 537-P.
16. Raz I, Hanefeld M, Xu L, Caria C, et al. Sitagliptin monotherapy improved glycemic control and beta-cell function after 18 weeks in patients with type 2 diabetes (T2DM) [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13 2006; Washington, DC. Abstract 1996-PO.
17. Aschner P, Kipnes M, Lunceford J, Mickel C, Davies MJ, Williams-Herman D. Sitagliptin monotherapy improved glycemic control in the fasting and postprandial states and beta-cell function after 24 weeks in patients with type 2 diabetes (T2DM) [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13 2006; Washington, DC. Abstract 1995-PO.
18. Mu J, Zhou Y, Woods J, Li Z, et al. Direct comparison of efficacy and durability of DPP-4 inhibitor, PPAR? agonist and sulfonylurea on glycemic control and B-cell function in a rodent model of type 2 diabetes. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13 2006; Washington, DC. Abstract 588-P.
19. Brazg R, Thomas K, Zhao P, Xu L, Chen X, Stein P. Effect of adding MK-0431 to on-going metformin therapy in type 2 diabetic patients who have inadequate glycemic control on metformin [abstract]. Presented at: American Diabetes Association 65th Annual Scientific Sessions; June 10–14, 2005; San Diego, Calif. Abstract 11-OR.
20. Karasik A, Charbonnel B, Liu J, Wu M, Meehan A, Meininger G. Sitaglipitin added to ongoing metformin therapy enhanced glycemic control and beta-cell function in patients with type 2 diabetes. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13, 2006; Washington, DC. Abstract 501-P.
21. Rosenstock J, Brazg R, Andryuk PJ, McCrary Sisk C, Lu K, Stein P. Addition of sitagliptin to pioglitazone improved glycemic control with neutral weight effect over 24 weeks in inadequately controlled type 2 diabetes (T2DM) [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13, 2006; Washington, DC. Abstract 556-P.
22. Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72.
23. Stevens C, Van Dyck K, Yi B, et al. Single doses of MK-0431, an inhibitor of dipeptidyl peptidase-IV, raise active GLP-1 levels without causing hypoglycemia in healthy subjects [abstract]. Presented at: American Diabetes Association 65th Annual Scientific Sessions; June 10–14, 2005; San Diego, Calif. Abstract 493-P.
24. Herman GA, Bergman A, Fang L, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of MK-0431 over 28 days in middle-aged, obese subjects [abstract]. Presented at: American Diabetes Association 65th Annual Scientific Sessions; June 10–14, 2005; San Diego, Calif. Abstract 497-P.
25. Scott R, Hartley P, Luo E, et al. Use of sitagliptin in patients with type 2 diabetes (T2DM) and renal insufficiency (RI) [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 6–13, 2006; Washington, DC. Abstract 1997-PO.
26. Wright D, Maes A, Yi B, et al. Multiple dose administration of MK-0431 (sitagliptin), an inhibitor of dipeptidyl peptidase-IV does not meaningfully alter the plasma pharmacokinetics or pharmacodynamics of single doses of warfarin [abstract]. Clin Pharm Ther. 2006;79;P76. Abstract PIII-64.
27. Ruddy MK, Bergman AJ, Zheng W, et al. Sitagliptin, a DPP-IV inhibitor, does not inhibit the pharmacokinetics of sulfonylurea, glyburide [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13, 2006; Washington, DC. Abstract 558-P.
28. Herman G, Bergman A, Yi B, et al. Co-administration of MK-0431 and metformin in patients with type 2 diabetes does not alter the pharmacokinetics of MK-0431 or metformin [abstract]. Presented at: American Diabetes Association 65th Annual Scientific Sessions; June 10–14, 2005; San Diego, Calif. Abstract 2099-PO.
29. Mistry G, Bergman A, Luo W, et al. Effects of sitagliptin on the pharmacokinetics (PK) of rosiglitazone in healthy subjects [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 6–13, 2006; Washington, DC. Abstract 2001-PO.
30. Krishna R, Bergman AJ, Larson P, et al. Effect of a single cyclosporine A (Neoral) dose on the single-dose pharmacokinetics (PK) of sitagliptin (MK-0431), a dipeptidyl peptidase-IV inhibitor (DPP-IV), in healthy male subjects [abstract]. Clin Pharm Ther. 2006;79;P63. Abstract PIII-17.
31. Bergman AJ, Cote J, Maes A, et al. Sitagliptin (MK-0431), a selective dipeptidyl-peptidase-IV (DPP-IV) inhibitor, does not affect the pharmacokinetics of simvastatin in humans [abstract]. Clin Pharm Ther. 2006;79;P48. Abstract PII-46.
32. Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72.
