- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Sitaxsentan: A new endothelin-receptor antagonist for the treatment of pulmonary arterial hypertension

Key Points
• Abstract
Elevated blood pressure in the pulmonary vasculature, known as pulmonary arterial hypertension (PAH), stresses the right side of the heart and can increase the risk for functional disability and death. In advanced disease, several drugs can be employed, including calcium-channel blockers, phosphodiesterase-5 inhibitors, prostacyclin analogues, and endothelin (ET)-receptor antagonists. ET is an endogenous peptide vasoconstrictor that is present in excessive concentrations in patients with PAH. Sitaxsentan, an ET-receptor antagonist undergoing FDA review, is selective for the ETA receptors. By selectively antagonizing the ETA receptors, sitaxsentan causes pulmonary arterial vasodilation and allows normal functioning of the ETB receptor, thus reducing the effects of ET. Clinical trials have demonstrated that orally administered sitaxsentan improves both 6-minute walking distance and World Health Organization functional class in patients with PAH. Trials have also demonstrated that sitaxsentan is relatively safe, although cases of hepatotoxicity have been reported. Further studies are necessary to better demonstrate the adverse event profile of this agent and to determine how sitaxsentan could be integrated into therapy for patients with PAH. (Formulary. 2007;42:295–301.)
Pulmonary arterial hypertension (PAH) is a disease characterized by vasoconstriction and overproliferation of the pulmonary arterial endothelium; these factors can stress the right side of the heart and increase the risk for functional disability and death.1 This disease can be insidious early on because measurement of systemic blood pressure (obtained via a blood pressure cuff on the arm) does not identify those with elevated pulmonary arterial pressures. Only more advanced hemodynamic monitoring, such as echocardiography or the insertion of a pulmonary artery catheter, can be used to determine pulmonary arterial pressure.2 In a patient without PAH, the pulmonary arterial pressure ranges from 12 to 16 mmHg at rest, whereas a patient with PAH has a pressure >25 mmHg at rest and >30 mmHg during exercise.2
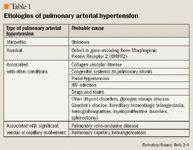
Patients with PAH have a poor prognosis, with an average survival time of <3 years from diagnosis. Inadequate cardiac output is usually associated with symptoms such as fatigue, dyspnea, atypical chest pain, and presyncope or syncope with exertion.2 With the onset of right-sided heart failure, patients may exhibit peripheral edema, jugular venous distension, and hepatojugular reflux.5 Signs of PAH include right ventricular hypertrophy on echocardiography or electrocardiography, and in advanced stages, the development of a third heart sound.2


Another pharmacologic treatment option for patients with PAH involves antagonism of endothelin (ET) receptors. ET is a peptide composed of 21 amino acids; this peptide is produced in endothelial cells. ET causes vasoconstriction and hypertrophy and has been demonstrated to be elevated in patients with PAH.5 Stimulation of ETA receptors appears to cause vasoconstriction and hypertrophy, whereas stimulation of ETB receptors may decrease the production of ET and increase its clearance.1 Bosentan, an ET-receptor antagonist approved by FDA for the treatment of PAH, antagonizes both ETA and ETB receptors, causing vasodilation and antiproliferation. Sitaxsentan (Thelin, Encysive) is a highly selective ETA-receptor antagonist currently under review by FDA for the treatment of PAH.
The choice of treatment for PAH is based on several factors. Bosentan and sildenafil have advantages compared with the prostacyclin analogues in that these agents are orally available, whereas iloprost, epoprostenol, and treprostinil require inhaled, intravenous, and subcutaneous administration, respectively. In patients with WHO class II PAH who are not candidates for therapy with calcium-channel blockers, other therapeutic modalities may be effective, but limited data are available and no specific drug can be recommended.6 In patients with WHO class III PAH, bosentan and epoprostenol have the strongest (level A) recommendation for use.6 In WHO class IV disease, epoprostenol has a level A recommendation, whereas bosentan has only a level B recommendation.6 Further studies are necessary to determine which level of recommendation sitaxsentan could be granted for the treatment of PAH once the drug receives FDA approval.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
