- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Teduglutide: A novel recombinant analog of human glucagon-like peptide-2 for short bowel syndrome
Teduglutide is a recombinant analog of human glucagon-like peptide-2 that is awaiting FDA approval for the treatment of short bowel syndrome (SBS) as a once-daily subcutaneous injection.
Abstract
Teduglutide is a recombinant analog of human glucagon-like peptide-2 that is awaiting FDA approval for the treatment of short bowel syndrome (SBS) as a once-daily subcutaneous injection. It has been shown to slow gastric emptying, reduce gastric secretions, increase intestinal blood flow, and stimulate growth of the small bowel and colon; teduglutide represents a major improvement in the treatment of SBS given that existing therapies are limited. Clinical trials have shown its efficacy in reducing the volume of parenteral support as either intravenous fluids (IVF) or parenteral nutrition (PN) and the number of days it is required weekly for SBS patients compared to placebo. Clinical trials have also shown it may allow SBS patients with their colon in continuity the option of stopping IVF or PN when used for up to 6 months. The long-term safety of teduglutide, however, requires further evaluation because of its potential to augment dysplastic transformation of intestinal tissue. (Formulary. 2012; 47:314–318.)
Short bowel syndrome (SBS) is characterized by malabsorption of macronutrients, electrolytes, vitamins, and trace elements that frequently leads to malnutrition, volume depletion, and weight loss. The most common conditions associated with SBS include surgical resections of the small bowel (and often colon) in Crohn's disease, mesenteric infarction, congenital anomalies, and cancer.1 Although there is heterogeneity in each patient based on their underlying disease(s), the remaining functionality and anatomy of their remnant bowel determines if they can sustain themselves with an oral diet. Most of these patients will require intermittent versus daily intravenous fluid (IVF) replacement and/or long-term parenteral nutrition (PN) to adequately nourish themselves. These therapies are expensive and frequently alter their quality of life with complications including catheter-related bloodstream infections and sepsis, loss of venous access, and possibly liver failure.2
Teduglutide (Gattex, NPS Pharmaceuticals) is a synthetic analogue of human GLP-2 that received FDA orphan drug designation for the treatment of SBS in 2000. It is currently at FDA as a new drug application and is the first new drug seeking approval for the treatment of SBS since 2003.1
CHEMISTRY AND PHARMACOLOGY
The discovery of the intestinotrophic hormonal effects of GLP-2 occurred in 1971 through findings from a case report of a patient with a glucagonoma who was experiencing small intestinal hyperplasia.4 The patient had a dilated small intestine with coarse, thickened mucosal folds, slowed intestinal transit (14 hr for barium to reach the patient's colon), and a marked increase in the caliber of the jejunum, all believed to be the result of excess glucagon from the patient's kidney tumor. Human GLP-2 is a 33-amino acid peptide that is located at the carboxy-terminal end of proglucagon.2,5 The specific G-protein coupled GLP-2 receptors are localized to enteroendocrine cells in the stomach, small intestine, and colon (L cells), as well as in the lung and central nervous system.1 Dietary fiber, short-chain fatty acids, carbohydrates, and fats are all potent stimulators of GLP-2 secretion in animals and humans.2
Teduglutide is a recombinant peptide analogue of human GLP-2 that contains a single amino acid substitution (glycine for alanine) at the N terminal that prevents enzymatic degradation by the proteolytic enzyme dipeptidyl peptidase-4 (DPP-4), thus prolonging its half-life and enhancing binding to the GLP-2 receptors.1 Teduglutide is recombinantly produced in Escherichia coli.
PHARMACOKINETICS
Teduglutide has a reported bioavailability of 86.5% to 89.2% after subcutaneous (SC) injection in the arm, thigh, and abdomen of healthy individuals for doses ranging from 10 mg to 80 mg (highest dose studied in humans).6 Following SC administration in the abdomen, however, absorption is approximately 50% faster than that observed in the arm or thigh.5 Peak plasma concentrations occur approximately 4 hours (range, 2.3-6 hr) after SC administration and the maximum plasma concentration after a 10-mg dose is 101±38.1 ng/mL.5,6 A 20-mg/mL formulation has higher peak plasma concentrations than a 50-mg/mL formulation, and the peak concentrations are 12% to 31% higher following SC administration in the abdomen. The clearance is 0.155 to 0.159 L/kg per hour in male and female subjects with 1-compartment linear elimination. The volume of distribution (Vd) is 32.8 L and, assuming an 87% bioavailability, a typical patient weighing 70 kg would have a Vd of 28.5 L.5 The elimination half-life ranges from 0.897 to 5.53 hours (vs 7 min for endogenous GLP-2) with minimal drug accumulation following repeated once-daily SC administration.5,6 GLP-2 and teduglutide are eliminated primarily via the kidneys. No dosage adjustment is necessary for patients with mild-to-moderate renal or liver impairment, and few patients have been studied with severe impairment of either of these organ systems to determine if dosage adjustment is needed. Dosages up to 0.15 mg/kg/day administered SC either once or twice daily have been studied in SBS patients.3 Specific information on the metabolism of teduglutide is limited in vivo, because it is likely degraded by DPP-4 to an inactive isomer similar to endogenous GLP-2.
CLINICAL TRIALS
Clinical studies of teduglutide in SBS would be expected to have greater results in patients with residual ileum and an intact colon given the abundance of L cells with the capacity to produce GLP-2 in these areas. Unfortunately SBS patients often have an end jejunostomy with no colon, leading to the most difficult circumstances for intestinal adaptation because of the lack of functional L cells and reduced or absent GLP-2.2
Phase 2 clinical trial. A phase 2 international, multicenter, open-label, dose-ranging, pilot trial evaluated 16 patients with SBS including 10 with an end jejunostomy (40-150 cm of remnant small intestine), 5 with 50% or more of colon in continuity (25-145 cm of remnant small intestine), and 1 patient with a jejunostomy and less than 50% (estimated 30%) of colon remaining.3 The patients eligible were adults (>18 years) without surgical resection of their stomach, duodenum, or pancreas and had a diagnosis of SBS from Crohn's disease (75%), mesenteric infarction, intestinal stricture, or volvulus. The SBS patients with 50% or more of colon in continuity had fecal weights exceeding 1 kg daily and fecal energy losses of 478 kcal daily. These 5 patients also had prestudy fasting native GLP-2 plasma concentrations determined. Each patient underwent a 72-hour nutrient balance (measuring all oral intake, fecal/stomal, and urine outputs) and a D-xylose absorption study (for intestinal carbohydrate absorption) with endoscopy/colonoscopy to determine the intestinal mucosa integrity and for biopsy samples at baseline, days 18 to 21, and after 3 weeks of follow-up. SBS patients with an end jejunostomy received teduglutide SC doses of 0.03 (n=2), 0.10 (n=5), and 0.15 (n=3) mg/kg/day, whereas those with 50% or more of colon in continuity received 0.10 mg/kg/day (n=5). The patient with a jejunostomy and less than 50% of colon remaining received 0.03 mg/kg/day for 21 days in the abdomen or thigh. The usual medications of the SBS patients (including proton-pump inhibitors, octreotide, codeine, loperamide, and oral supplements) were kept constant during the 21-day trial of teduglutide. Interestingly, 5 patients from the highest dosing groups (0.10 and 0.15 mg/kg/d) were rechallenged at least 3 months after their initial participation to investigate antibody formation because teduglutide was recombinantly produced from E. coli and to evaluate twice-daily dosing (0.05 or 0.075 mg/kg every 12 hr).3
The 21-day treatment with teduglutide significantly decreased fecal wet weight compared with baseline in all patients (P<.05), including those with an end jejunostomy and those with 50% or more of colon in continuity. The increase in relative absorption was from 20% to 26% (means) depending on the colon being in continuity, which was slightly more than that for end jejunostomy patients (P<.03). Additionally urine output increased in 14 of 16 SBS patients reflecting greater absorption of oral intake (P<.008). Villus height, crypt depth, and mitotic index of the intestinal mucosa biopsies significantly improved in the SBS patients with an end jejunostomy (P<.04). Body weight increased by a mean of 0.9 kg in 11 of 16 SBS patients during the 21-day treatment, although it was not statistically significant (P=.12), most likely due to the small number of patients in this trial. The fasting concentrations of endogenous GLP-2 for the 5 SBS patients with 50% or more of colon in continuity were closer to the fed state for healthy fasting volunteers in 4 of the 5 patients.3
Phase 3 clinical trials. The first phase 3 international, multicenter, randomized, double-blind, placebo-controlled trial evaluated 83 patients with SBS receiving teduglutide SC once daily (in the abdomen or thigh) as 0.05 mg/kg/day (n=35), 0.10 mg/kg/day (n=32), or placebo (n=16) over a 24-week period.7 After an 8-week optimization period of IVF or PN, all SBS patients had a 4- to 8-week stabilization period prior to randomization to optimize their IVF or PN to maintain a urine output of 1 to 2 L daily while on a consistent oral diet. The randomization was stratified into 3 groups based on the following: IVF only 3 to 7 times weekly, PN administered 3 to 5 times weekly, and PN administered 6 to 7 times weekly. The primary outcome in this trial was the percentage of SBS patients who had a reduction from baseline in IVF or PN volume of 20% to 100% at week 16 of treatment and again at weeks 20 and 24. Secondary outcomes included the number and percentage of SBS patients who responded with a 20% or more reduction in IVF or PN at week 20 of treatment and again at week 24, absolute reduction from baseline in IVF or PN, achievement of at least a 1-day reduction in weekly IVF or PN administration, and total weaning from IVF or PN. Additionally, all of the SBS patients completing the 24-week placebo-controlled trial were offered active treatment in a 28-week extension trial.7
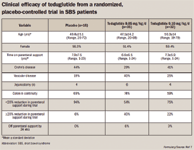
The second phase 3 international, multicenter, randomized, double-blind, placebo-controlled trial (STEPS, Study of Teduglutide in PN-dependent Short-bowel syndrome) evaluated 86 patients with SBS (78 completed the trial) receiving teduglutide SC once daily as 0.05 mg/kg/day (n=39) or placebo (n=39) over a 24-week period.8 After an undefined optimization/stabilization period of IVF or PN, all SBS patients were randomized. The primary outcome in this trial was the percentage of SBS patients who had a reduction from baseline in IVF or PN volume of 20% to 100% at weeks 20 and 24.
The intention-to-treat population (n=86) showed 63% of teduglutide patients had a reduction from baseline in IVF or PN volume of 20% to 100% at weeks 20 and 24 versus 30% of placebo patients (P=.002). At 24 weeks, the mean IVF or PN volume reduction for teduglutide was statistically significant when compared with placebo (–4.4 L/wk vs –2.3 L/wk, P=.001). Additionally, significantly more teduglutide patients were able to reduce the number of infusion days per week by 1 or more days compared with placebo patients (54% vs 23%, P=.0047).8
The STEPS 2 trial is an international, multicenter, open-label trial of SC teduglutide 0.05 mg/kg/day enrolling the original STEPS (treated and placebo) patients over a 2-year period to determine long-term safety and tolerability. Preliminary results at 6 months are available from this trial with 76 of the original 78 STEPS patients enrolling.9 Interestingly there have been 3 SBS patients (2 previous teduglutide-treated STEPS patients and 1 placebo STEPS patient now receiving teduglutide) completely weaned from their IVF or PN in this portion of the trial.9
Adverse Events
In the phase 2 clinical trial, the most common adverse effects were lower extremity edema (44% of patients), localized swelling of the jejunostomy stoma (70% of patients with an end jejunostomy), headache (25% of patients), and abdominal pain (19% of patients). Only 19% experienced a minor injection site reaction (bruising, induration, or rash) and no antibodies to teduglutide were detected.3
In the first phase 3 clinical trial, 95% of the 83 SBS patients experienced at least 1 adverse effect with 1 in the placebo group, 6 in the 0.05 mg/kg/day group, and 2 in the 0.10 mg/kg/day group discontinuing the trial because of significant adverse effects.7 The most common adverse effects in the teduglutide treatment arms were abdominal pain (24%), headache (24%), nausea (22%), nasopharyngitis (16%), and vomiting (15%).7 During the histopathologic evaluation, no intestinal tissue samples displayed dysplastic transformation from the biopsies (optionally taken from stomas or by colonoscopy).7
In the STEPS trial, the number of SBS patients discontinuing the trial was 3 in the placebo group and 2 in the 0.05 mg/kg/day teduglutide group because of adverse effects. The most common adverse effects were abdominal pain, nausea, gastrointestinal (GI) stoma complications, and abdominal distention.8
In the STEPS 2 trial, 12 patients have been discontinued from the trial (9 of them for adverse effects) to date. The most common adverse effects reported are GI (22%, including abdominal pain, distention, nausea, and vomiting), generalized disorders (13%, including edema, injection site-related events, and pyrexia) and infection (12%, including catheter-related, urinary tract, and viral infections). No SBS patients have been identified as having neutralizing antibodies to teduglutide.9
DRUG INTERACTIONS
Currently, only limited data are available regarding potential drug interactions with teduglutide. Most SBS patients in the previously mentioned trials were maintained on their oral medication regimens consisting mostly of histamine2 receptor antagonists, proton-pump inhibitors, bile-sequestering agents, octreotide, codeine and other opiates, loperamide, diphenoxylate/atropine, and oral glutamine without reported problems.
DOSING AND ADMINISTRATION
Clinical trials have evaluated teduglutide administered SC either once or twice daily at doses of 0.03, 0.05, 0.10, and 0.15 mg/kg/day for up to 24 weeks.3,7,8 The STEPS 2 trial will extend this dosing for the group receiving 0.05 mg/kg/day to 2 years when it is completed.9 Patients should be monitored for injection site-related reactions (bruising, induration, erythema, rash, pain) and if they have an ostomy, the stomal opening should be evaluated for potential closure or swelling. GI symptoms are to be expected and may preclude patients from continuing therapy long term with SBS, as many of them already experience these from their underlying disease(s). Patients with a history of abdominal pain and recurrent small bowel obstructions should use teduglutide with caution.7 Teduglutide is renally eliminated; however, dosage adjustment in mild-to-moderate renal insufficiency is not recommended. It has been administered to SBS patients with mild-to-moderate hepatic impairment (Child-Pugh score 7-9) without reported problems.5 The need for dosage adjustment in severe renal and hepatic impairment remains unknown.
FORMULARY CONSIDERATIONS
Teduglutide is the first GLP-2 analogue awaiting approval from FDA for the treatment of SBS. It has been shown to reduce the need for IVF and/or PN with use up to 6 months in SBS patients. It therefore represents a major benefit for these unfortunate patients. There is also the dramatic potential for it to completely allow SBS patients to remain off PN given the long-term risk of hepatic failure with prolonged PN and the venous access issues these patients frequently encounter. The long-term safety with teduglutide remains to be determined beyond 6 months, and the development of dysplastic transformation in intestinal tissue continues to be a potential risk.10 The implementation of teduglutide will need to occur under the close supervision of a physician trained in the management of SBS and PN therapy or under the supervision of a nutrition support team within an institution or home care company. Postmarketing studies should evaluate the occurrence of cholelithiasis, biliary pancreatitis, and liver dysfunction with its long-term use given that most SBS patients are already at increased risk for these conditions.10 The optimal dosage will likely be in the range of 0.05 to 0.15 mg/kg/day, with acquisition costs yet to be determined for the medication. Patients with inflammatory bowel disease may also be a unique subset of patients for whom teduglutide may represent a therapeutic option for remission or mucosal healing.
Dr Canada is clinical pharmacy services manager and nutrition support team coordinator at University of Texas MD Anderson Cancer Center, Houston, Texas; adjunct assistant professor at University of Texas at Austin College of Pharmacy, Austin; and adjunct clinical assistant professor at University of Houston College of Pharmacy, Houston.
Disclosure Information: The author reports no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, edical editor, University of Connecticut/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn., and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Vipperla K, O'Keefe SJ. Teduglutide for the treatment of short bowel syndrome. Expert Rev Gastroenterol Hepatol. 2011;5:665–678.
2. Tee CT, Wallis K, Gabe SM. Emerging treatment options for short bowel syndrome: Potential role of teduglutide. Clin Exp Gastroenterol. 2011;4:189–196.
3. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–1231.
4. Gleeson MH, Bloom SR, Polak JM, Henry K, Dowling RH. Endocrine tumour in kidney affecting small bowel structure, motility, and absorptive function. Gut. 1971;12:773–782.
5. Marier JF, Mouksassi MS, Gosselin NH, et al. Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn's disease. J Clin Pharmacol. 2010;50:36–49.
6. Marier JF, Beliveau M, Mouksassi MS, et al. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol. 2008;48:1289–1299.
7. Jeppesen PB, Gilroy R, Pertkiewicz M, et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914.
8. O'Keefe S, Jeppesen P, Pertkiewicz M, et al. Teduglutide, a novel analog of glucagon-like peptide 2 (GLP-2), is effective and well tolerated in reducing parenteral support (PS) volume in short bowel syndrome-intestinal failure (SBS-IF) subjects: Results from a 24-week, placebo-controlled phase 3 trial [abstract 247]. Am J Gastroenterol. 2011;106(Suppl 2):S97.
9. Schwartz L, Seidner D, Jeppesen P, et al. Teduglutide (TED) for the treatment of short bowel syndrome-intestinal failure (SBS-IF) subjects yields further reductions in parenteral support (PS): An interim assessment of a 2-year, open-label, phase 3 trial (STEPS2) [abstract 251]. Am J Gastroenterol. 2011;106(Suppl 2):S99.
10. Nørholk LM, Holst JJ, Jeppesen PB. Treatment of adult short bowel syndrome patients with teduglutide. Expert Opin Pharmacother. 2012;13:235–243.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
