- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Telavancin: A novel lipoglycopeptide antibiotic with dual mechanisms of action
Telavancin is a novel semisynthetic lipoglycopeptide antibiotic undergoing FDA review for complicated skin and skin-structure infections; this agent is also in phase 3 clinical trials for the treatment of hospital-acquired pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) or multidrug-resistant Streptococcus pneumoniae. Telavancin exerts its antibacterial action via a dual mode of action involving both inhibition of peptidoglycan synthesis and disruption of the bacterial cell membrane; the latter of these effects is believed to improve the rate of killing observed with telavancin compared with other glycopeptides. In vitro, telavancin exhibits good activity against a variety of gram-positive organisms, including drug-resistant S pneumoniae, MRSA, vancomycin intermediate-susceptible and vancomycin-resistant S aureus, and vancomycin-resistant enterococci. Clinical data have demonstrated that telavancin is at least as effective as comparator agents for a variety of infectious processes...

Key Points
Abstract
Over the past few decades, resistant bacteria have continued to increase in prevalence and significance. The historical gold standard for the treatment of infections caused by resistant gram-positive pathogens has been vancomycin. Unfortunately, contemporary use of vancomycin has been tempered by increasing rates of resistance, leading researchers to investigate other antimicrobial options. Telavancin is a novel semisynthetic lipoglycopeptide antibiotic undergoing FDA review for complicated skin and skin-structure infections; this agent is also in phase 3 clinical trials for the treatment of hospital-acquired pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) or multidrug-resistant Streptococcus pneumoniae. Telavancin exerts its antibacterial action via a dual mode of action involving both inhibition of peptidoglycan synthesis and disruption of the bacterial cell membrane; the latter of these effects is believed to improve the rate of killing observed with telavancin compared with other glycopeptides. In vitro, telavancin exhibits good activity against a variety of gram-positive organisms, including drug-resistant S pneumoniae, MRSA, vancomycin intermediate-susceptible and vancomycin-resistant S aureus, and vancomycin-resistant enterococci. Clinical data have demonstrated that telavancin is at least as effective as comparator agents for a variety of infectious processes. In general, this agent appears to be well tolerated, with patients typically experiencing relatively mild adverse events. Telavancin has the potential to become a much-needed addition to the antimicrobial arsenal. (Formulary. 2007;42:545–557.)
Resistant gram-positive pathogens such as drug-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin intermediate-susceptible and vancomycin-resistant S aureus (VISA and VRSA, respectively), and vancomycin-resistant enterococci (VRE) represent formidable therapeutic challenges for clinicians. Over the past few decades, these resistant bacteria have continued to increase in prevalence and significance. It has been estimated that there are approximately 125,969 hospitalizations annually for a diagnosis of infection secondary to MRSA.1 MRSA accounts for an estimated 12% of all nosocomial bacteremias, 28% of surgical wound infections, and 21% of nosocomial skin infections.2 Infections secondary to MRSA result in excess costs of approximately $4,000 per patient per hospitalization compared with patients infected with methicillin-susceptible S aureus (MSSA).3
The historical gold standard for the treatment of infections caused by resistant gram-positive pathogens has been vancomycin. Unfortunately, contemporary use of vancomycin has been tempered by increasing rates of resistance among pathogens such as S aureus and Enterococcus species. Furthermore, clinicians have become concerned about the relatively slow rates of kill noted with vancomycin compared with other agents such as the beta-lactams and the possible implications of this slow kill rate for treatment outcomes.4,5 Additionally, the need for therapeutic drug monitoring with vancomycin and lingering concerns regarding possible drug-related nephrotoxicity and infusion-related adverse events have left clinicians searching for an acceptable therapeutic alternative. During the past decade, several new agents have been developed in an effort to combat the growing problem of resistance among gram-positive pathogens. One agent currently in development is telavancin (Theravance). Telavancin is a novel lipoglycopeptide antibiotic that is undergoing FDA review for complicated skin and skin-structure infections; this agent is also in phase 3 clinical trials for the treatment of hospital-acquired pneumonia caused by MRSA or multidrug-resistant S pneumoniae.
CHEMISTRY AND PHARMACOLOGY

Telavancin and other new lipoglycopeptide derivatives, such as dalbavancin and ortivancin, were designed to provide broad coverage against gram-positive organisms and to address the escalating problems of VRE and MRSA. It is believed that the improved activity noted with telavancin may be the result of a dual mechanism of action exerted against gram-positive bacterial species.8 Like the glycopeptides, telavancin inhibits microbial growth by binding to the D-Ala-D-Ala moiety of cell wall precursors, thereby disrupting peptidoglycan synthesis. However, the level of telavancin's antimicrobial activity has not been correlated with the degree of peptidoglycan inhibition via this means, thus suggesting the existence of an additional mechanism of action.7
Higgins et al6 sought to elucidate the molecular mechanism of the antibacterial action of telavancin. The investigators compared the degree of inhibition of peptidoglycan synthesis and the effects on membrane potential and permeability elicited by telavancin, vancomycin, and THRX-881620 (a hexapeptide analog of telavancin deficient of the N-terminal amino acid in the carboxylate binding pocket). The investigators noted that despite having approximately 5-fold reduced affinity for the D-Ala-D-Ala moiety compared with vancomycin, telavancin was approximately 10 times more potent at inhibiting peptidoglycan synthesis than vancomycin. Furthermore, the researchers observed that telavancin was largely bound to the D-Ala-D-Ala moiety of peptidoglycan within the cell membrane, whereas vancomycin binds primarily to moieties located within the cell wall. A second mechanism of action was also observed with telavancin: perturbation of bacterial cell permeability, resulting in leakage of intracellular adenosine-5'-triphosphate (ATP) and potassium, thereby leading to altered membrane potential. The authors suggested that telavancin-induced changes in the cell membrane were responsible for the rapid concentration-dependent bactericidal activity noted with telavancin.
Telavancin's dual mechanism of action is of great importance not only because of the rapid antibacterial action afforded, but also because this mechanism may help to deter the development of resistance to telavancin. Sahm et al9 observed a low potential for telavancin-induced resistance in vitro among several clinically important gram-positive species, including MSSA, Staphylococcus epidermidis (methicillin-resistant, vancomycin-resistant, and daptomycin-nonsusceptible), VRE, and multidrug-resistant Streptococcus species.
PHARMACOKINETICS
As part of a 2-phase, dose-ranging study, Shaw et al10 analyzed the pharmaco-kinetic profile of telavancin in a group of healthy male volunteers after administration of single and multiple infusions. In the first phase of this study, participants received telavancin via a single intravenous (IV) infusion at doses ranging from 0.25 to 15 mg/kg. After this infusion, telavancin exhibited a brief distribution phase followed by a monoexponential elimination phase. The investigators noted that telavancin exhibited linear pharmacokinetics at doses ≤12.5 mg/kg; however, at doses higher than this, a dose-proportional increase in maximum plasma concentration (Cmax) was noted. Additionally, the elimination half-life (t1/2) of telavancin increased as the dose was increased (range, 2.9 h after 0.25-mg/kg dosing–9.1 h after 15-mg/kg dosing).

Human and animal data suggest that telavancin exhibits a high degree (>90%) of protein binding.8,12 This characteristic makes knowledge of free drug concentrations at likely sites of infection critical. Sun et al13 evaluated the penetration of telavancin into the blister fluid of 9 healthy volunteers. The volunteers received three 7.5-mg/kg doses administered every 24 hours via a 60-minute infusion. After the last dose, the mean telavancin plasma concentration was 84.8 mcg/mL compared with a mean blister fluid concentration of 16.0 mcg/mL (steady-state AUC blister fluid:steady-state AUC plasma ~40%). The estimated free drug concentrations in the blister fluid exceeded the reported minimum inhibitory concentrations (MICs) of telavancin for MSSA, MRSA, penicillin-susceptible and penicillin-resistant S pneumoniae, and vancomycin-resistant Enterococcus faecalis. Penetration of telavancin into human pulmonary epithelial lining fluid and alveolar macrophages has also been described.14 After 3 daily doses of 10 mg/kg, mean total telavancin concentrations in the epithelial lining fluid of healthy volunteers exceeded 0.9 mcg/mL at all time points during the 24-hour sampling period. Of added significance was the observation that drug concentrations were consistently higher in alveolar macrophages than in the epithelial lining fluid at all times examined.

SPECTRUM OF ACTIVITY
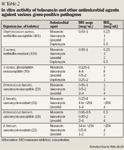
Telavancin has also demonstrated activity against a variety of gram-positive anaerobes, including Actinomyces, Clostridium (including Clostridium difficile), Eubacterium, Lactobacillus (diminished activity was noted against Lactobacillus casei), Propionibacterium, Peptostreptococcus anaerobius, and Corynebacterium.23
CLINICAL TRIALS
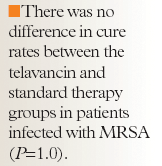
Preliminary results from the Assessment of Telavancin in Skin and Skin Structure Infections (ATLAS 1) trial, a large, randomized, double-blind, phase 3 study, demonstrated the noninferiority of telavancin (10 mg/kg once daily) compared with vancomycin (1 g every 12 h) for the treatment of gram-positive skin and soft-tissue infections in 855 patients.26 Response rates among clinically evaluable patients were observed to be similar in the telavancin-treated and vancomycin-treated groups (87.9% vs 86.5%, respectively). Additionally, no difference was observed between the telavancin-treated and vancomycin-treated groups with respect to microbiologic cure rates among evaluable patients and in the MRSA subset (89.5% vs 85.9% and 87.1% vs 84.8%, respectively).
A second phase 3 trial comparing the efficacy of telavancin and vancomycin for the treatment of complicated skin and skin-structure infections, the ATLAS 2 trial, also demonstrated the noninferiority of telavancin.27 In this trial, which included 1,012 patients, cure among clinically evaluable patients was observed in 88.7% of patients treated with telavancin and in 87.6% of patients treated with vancomycin. Furthermore, similar rates of microbiologic eradication were observed in evaluable patients and in the MRSA subset of patients. However, when an end point of overall therapeutic response (cure plus eradication) was assessed in evaluable patients, patients treated with telavancin achieved a higher response than those treated with vancomycin (92% vs 84.7%, respectively).
Telavancin is currently in phase 3 trials for the treatment of hospital-acquired pneumonia due to gram-positive bacteria, including resistant strains such as MRSA.
ADVERSE EVENTS
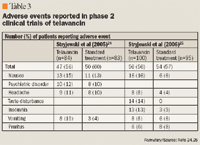
Preclinical in vitro hERG assay data and early clinical data have demonstrated that treatment with telavancin may prolong the QTc interval when administered at doses >7.5 mg/kg. In 1 of the phase 2 trials, mean QTc wave length was 12.5 ms longer in patients receiving telavancin 10 mg/kg once daily compared with patients receiving standard therapy consisting of vancomycin, nafcillin, oxacillin, or cloxacillin (P≤.0001).25 In the other phase 2 trial, a 6.4-ms prolongation in the QTc interval was observed among patients receiving telavancin 7.5 mg/kg versus those receiving standard therapy.24 There were no documented adverse cardiac events associated with QTc wave prolongation in either of the aforementioned trials.24,25 However, because of these observations, 160 patients were enrolled in a study designed to examine the effect of telavancin on QTc interval.28 Patients were randomized to receive either placebo, telavancin 7.5 mg/kg or 15 mg/kg, or moxifloxacin 400 mg. Placebo-corrected prolongations in QTc intervals of 4.1 msec, 4.5 msec, and 9.2 msec were noted with telavancin 7.5 mg/kg (P=.036), telavancin 15 mg/kg (P=.027), and moxifloxacin (P<.001), respectively. Although the clinical relevance of this mild cardiac effect appears to be negligible, continued evaluation of telavancin in clinical trials will provide further information about the importance of this finding.


DRUG INTERACTIONS
Information regarding clinically significant drug interactions with telavancin is not yet available.
DOSING AND ADMINISTRATION
The dosing regimen that will be recommended if telavancin is approved by FDA has not yet been established. However, doses of 7.5 to 10 mg/kg administered via IV every 24 hours have been evaluated in phase 2 trials and appear to be both efficacious and well tolerated.24,25 The dose of 10 mg/kg is being further evaluated in phase 3 studies.26,27
Dr Knechtel is a pharmacy practice resident, Northwestern Memorial Hospital, Chicago, Illinois. Ms Jacobs is a PharmD candidate, Purdue University School of Pharmacy, West Lafayette, Indiana. Dr Klepser is a professor, Borgess Medical Center, Ferris State University College of Pharmacy, Kalamazoo, Michigan.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant-Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11:868–872.
2. Baquero F. Gram-positive resistance: Challenge for the development of new antibiotics. J Antimicrob Chemother. 1997;39(suppl A):1–6.
3. Kopp BJ, Nix DE, Armstrong EP. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann Pharmacother. 2004;38:1377–1382.
4. Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34: 1227–1231.
5. Cantoni L, Glauser MP, Bille J. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob Agents Chemother. 1990; 34:2348–2353.
6. Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49: 1127–1134.
7. Pace JL, Judice JK. Telavancin (Theravance). Curr Opin Investig Drugs. 2005;6:216–225.
8. Hegde SS, Reyes N, Wiens T, et al. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob Agents Chemother. 2004;48:3043–3050.
9. Sahm DF, Benton BM, Cohen MA, et al. Telavancin demonstrates a low potential for in vitro selection of resistance among key target gram-positive species [abstract]. Presented at: 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 27–30, 2006; San Francisco, CA. Abstract C1-681.
10. Shaw JP, Seroogy J, Kaniga K, Higgins DL, Kitt M, Barriere S. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother. 2005;49:195–201.
11. Barriere S, Shaw JP, Seroogy J, Spencer E, Kitt M. Pharmacokinetics (PK) & safety of a new antibacterial, TD-6424 (TD), in healthy subjects [abstract]. Presented at: 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–, 2003; Chicago, IL. Abstract A-20.
12. Kaniga K, Johnston D, Wu T, et al. Effect of human serum on time-kill curves, minimum inhibitory and bactericidal concentrations of TD-6424 against staphylococci and enterococci [abstract]. Presented at: 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, IL. Abstract C1-1632.
13. Sun HK, Duchin K, Nightingale CH, Shaw J-P, Seroogy J, Nicolau DP. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob Agents Chemother. 2006; 50:788–790.
14. Gotfried M, Duchin K, Shaw J, et al. Telavancin (TLV) penetrates well into human pulmonary epithelial lining fluid (ELF) and alveolar macrophages (AM) and is unaffected by pulmonary surfactant [abstract]. Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract A-14.
15. Duchin K, Shaw J, Spencer E, Seroogy J, Barriere S, Wilbraham D. Single dose pharmacokinetics (PK) of telavancin (TLV) in healthy elderly subjects [abstract]. Presented at: 14th European Congress of Clinical Microbiology and Infectious Diseases; May 1–4, 2004; Prague, Czech Republic. Abstract P1027.
16. Duchin K, Shaw J, Spencer E, Seroogy J, Barriere S, Wilbraham D. Single dose pharmacokinetics (PK) of telavancin (TLV) in subjects with renal dysfunction [abstract]. Presented at: 14th European Congress of Clinical Microbiology and Infectious Diseases; May 1–4, 2004; Prague, Czech Republic. Abstract P1028.
17. Wong SL, Shaw JP, Bereriere S, Kitt M, Goldberg M. Pharmacokinetics of intravenous telavancin in subjects with hepatic impairment [abstract]. Presented at: 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 27–30, 2006; San Francisco, CA. Abstract A-1951.
18. Draghi DC Jones ME, Flamm RK, Thornsberry C, Sahm DF. Telavancin activity against current and diverse populations of enterococci and Streptococcus pneumoniae [abstract]. Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract E-1747.
19. Leuthner KD, Cheung CM, Rybak MJ. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2006;58:338–343.
20. King A, Phillips I, Kaniga K. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration-dependent anti-infective with multiple mechanisms of action against gram-positive bacteria. J Antimicrob Chemother. 2004;53:797–803.
21. Laohavaleeson S, Kuti JL, Nicolau DP. Telavancin: A novel lipoglycopeptide for serious gram-positive infections. Expert Opin Investig Drugs. 2007;16:347–357.
22. Draghi DC, Jones ME, Thornsberry C, Flamm RK, Sahm DF. Telavancin activity against current and diverse Staphylococcus aureus populations [abstract]. Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract E-1744.
23. Goldstein EJ, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents against anaerobic gram-positive species and Corynebacterium spp. Antimicrob Agents Chemother. 2004;48:2149–2152.
24. Stryjewski ME, O'Riordan WD, Lau WK, et al. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis. 2005; 40:1601–1607.
25. Stryjewski ME, Chu VH, O'Riordan WD, et al. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother. 2006;5:862–867.
26. Corey GR, Stryjewski ME, O'Riordan WD, et al; for the ATLAS Study Group. ATLAS 1: The first phase 3 study evaluating the new lipoglycopeptide, telavancin, for the treatment of patients with complicated skin and skin structure infections. Presented at:17th European Congress of Clinical Microbiology and Infectious Diseases; March 31–April 4, 2007; Munich, Germany. Abstract 1733-343.
27. Corey GR, Stryjewski ME, Fowler VG Jr, et al; for the ATLAS Study Group. ATLAS 2: A double-blind, randomised, active controlled, multinational phase 3 study comparing telavancin with vancomycin for the treatment of patients with complicated skin and skin structure infections. Presented at:17th European Congress of Clinical Microbiology and Infectious Diseases; March 31–April 4, 2007; Munich, Germany. Abstract 1733-344.
28. Barriere S, Genter F, Spencer E, Kitt M, Hoelscher D, Morganroth J. Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J Clin Pharmacol. 2004;44:689–695.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
