- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
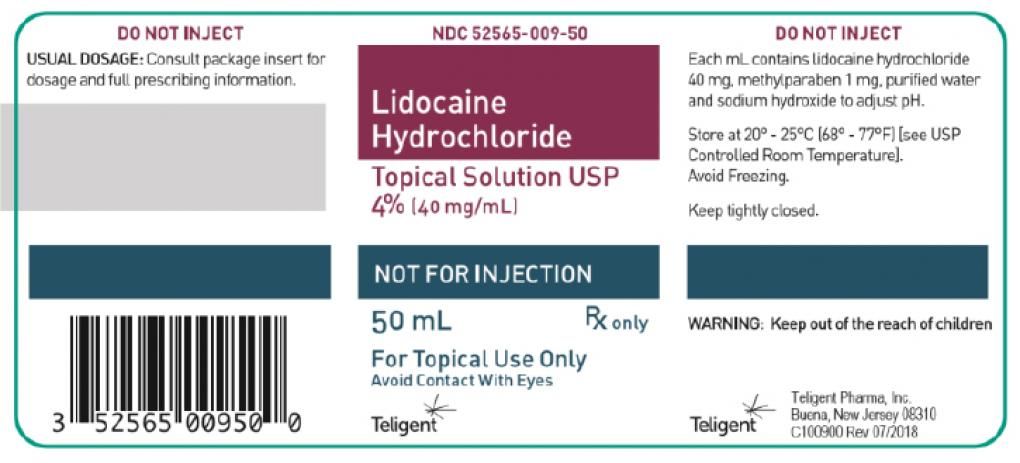
Teligent Recalls One Lot of Lidocaine
The product is considered “super potent” with a higher dose that could result in serious cardiac toxicities.
Teligent Pharma is voluntarily recalling one lot of lidocaine HCl topical solution 4%. The product is being recalled because the company’s testing has found it to be “super potent.”
The use of a super potent product would result in an increased dose, which could lead to the development of local systemic toxicity depending on the duration of the treatment and the specific patient.
The FDA warns that local anesthetic systemic toxicity can result in central nervous system reactions including excitation and/or depression and more serious signs of cardiovascular toxicity, such as bradycardia, hypotension, and even cardiovascular collapse can present very quickly. To date, company officials said they have not received any reports of adverse events.
The product is used for the production of topical anesthesia.
The product under recall is in a 50 ml glass bottle with a screw cap with the NDC number of 52565-009-50. The lot number is 14218, expiration 09/2022. The packaging for the recalled lot is below.
The product was distributed at the wholesale and retail distribution levels in the US and Canada.