- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
A timely transition to insulin: Identifying type 2 diabetes patients failing oral therapy
Although oral antidiabetic medications initially may be effective for controlling hyperglycemia, these agents often fail to maintain adequate glycemic control as the disease progresses, and insulin eventually is required in most patients. This review explores strategies for identifying patients with type 2 diabetes who are failing to maintain glycemic control on oral agents and for transitioning these patients to insulin. Based on available data, patients are not reaching recommended glycemic goals due to delays in and reluctance towards intensification of therapy, resulting in an increased risk of complications.


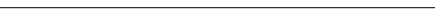
Type 2 diabetes is a progressive disease characterized by the dual defect of gradual declines in insulin secretion and insulin resistance.1 Both of these defects are present at the outset of disease in those destined to have type 2 diabetes. Insulin resistance is a disorder in which the body does not respond to or utilize insulin appropriately.2 While insulin resistance generally remains constant throughout the course of the disease, the β-cells of the pancreas gradually become unable to secrete enough insulin to overcome the degree of insulin resistance.2,3 As pancreatic β-cell function progressively deteriorates, some degree of absolute insulin deficiency develops, resulting in chronic hyperglycemia. As a result, essentially all patients who develop type 2 diabetes eventually have reduced levels of insulin secretion.4
Economic costs attributed to diabetes in 2002 were estimated at $132 billion in the United States alone.5 This estimate includes $91.8 billion in direct medical expenditures, of which $24.6 billion encompasses costs to treat chronic complications associated with the disease.5 These estimates likely underestimate the true cost of the disease, since expenditures related to intangible costs, such as pain and suffering as well as care provided by unpaid caregivers, are not included.5 An age-adjusted annual per capita expenditure for subjects without diabetes was calculated to be $5,642. This represents an approximately 2.4-fold increase in costs for subjects with diabetes. Because approximately 90% to 95% of all cases of diabetes are type 2, it may be presumed that type 2 diabetes accounts for the vast majority of these health-care costs.2
EARLY INTENSIVE GLYCEMIC CONTROL Glycemic control is fundamental to the prevention and management of diabetic complications.1,2 It often is assessed using A1C testing, a measure of mean glycemic control over the preceding 2 to 3 months.1 In an effort to explore the impact of early glycemic control on diabetic complications, the United Kingdom Prospective Diabetes Study (UKPDS) Group compared the effects of conventional (diet modification) and intensive (sulfonylurea or insulin therapy) blood glucose control on A1C levels and the risk for complications in patients with newly diagnosed type 2 diabetes.3 Over a 10-year treatment period, intensive treatment resulted in relative reduction of A1C levels by 11%, the risk for any diabetes-related end point by 12%, any diabetes-related death by 10%, and all-cause mortality by 6%, compared with conventional treatment. "Any diabetes-related end point" was defined by the UKPDS group as sudden death, death from hyperglycemia or hypoglycemia, fatal or non-fatal myocardial infarction, angina, heart failure, stroke, renal failure, amputation (of at least 1 digit), vitreous hemorrhage, retinopathy requiring photocoagulation, blindness in 1 eye, or cataract extraction.3
The American Diabetes Association (ADA) has recommended targeting A1C goals of <7.0% in patients with diabetes.1 ADA also has suggested that targeting A1C levels of <6.0% may be appropriate in some individual patients (eg, those without severe or frequent hypoglycemia) based on data showing that there is no limit of A1C below which further lowering does not reduce the risk of complications.1 Despite these recommendations, glycemic treatment goals still are not being met in a substantial proportion of patients with diabetes.11 Cross-sectional surveys conducted across the United States through the Third US National Health and Nutrition Examinations Survey (N=1,026) and the Behavioral Risk Factors Surveillance System (N=3,059) have found that 57% of individuals with diabetes have A1C levels >7.0%, 18% have A1C levels >9.5%, and 15% have A1C levels $10.0%.11 In another large study involving 20 public and private health organizations, 35% of all patients with diabetes evaluated had A1C levels >9.5%.12 With current goals for glycemic control remaining unmet in the majority of patients with diabetes, it is now appropriate to re-evaluate current approaches to the management of diabetes and their implementation in clinical practice.
Patients who are unable to achieve adequate glycemic control through lifestyle changes alone should be started on an oral antidiabetic; typically, this is either a sulfonylurea or metformin.13 While these agents are similarly effective in decreasing plasma glucose levels, metformin often is preferred in overweight patients with type 2 diabetes since it also promotes weight loss, does not cause hypoglycemia, and has been shown to have cardioprotective effects apparently independent of glucose lowering.3,14 Doses of oral antidiabetic agents are generally adjusted upward over a period of several weeks until glycemic goals or maximum doses are reached. Patients who fail to achieve glycemic control on monotherapy with a sulfonylurea or metformin should be started on a second oral antidiabetic agent from a different therapeutic class. Metformin plus a sulfonylurea is the most frequently used oral combination.15 Patients who are still unable to achieve target A1C goals of <7.0% while taking a combination of 2 oral antidiabetic medications require additional therapy with either a third oral antidiabetic agent, such as a thiazolidinedione, or insulin.16
Most patients with type 2 diabetes will eventually fail oral antidiabetic therapy, even those who initially respond to treatment. Monotherapy with a single oral antidiabetic agent is unlikely to maintain adequate glycemic control for more than a few years. In a study by the UKPDS Group, 53% of newly diagnosed patients with type 2 diabetes treated with sulfonylurea monotherapy required insulin over a period of 6 years.17 Although combination therapy with 2 oral antidiabetic medications is effective in lowering A1C levels by 3% to 4%, approximately 30% of patients remain unable to achieve target levels even when receiving maximum doses of oral agents. While the addition of a thiazolidinedione to combination therapy with metformin and a sulfonylurea has been shown to substantially further reduce A1C levels in some, many patients with diabetes appear to be unable to achieve and/or maintain glycemic control even on 3 oral antidiabetic agents.18 In a study of 35 patients with type 2 diabetes treated with a sulfonylurea, metformin, and a thiazolidinedione, 26% of patients were unable to maintain adequate glycemic control (A1C <7.0%), with mean A1C levels of 8.8%.18 The well-established association between uncontrolled hyperglycemia and the development of diabetic complications suggests that it is inappropriate to allow hyperglycemia to persist in patients who are no longer achieving glycemic control on oral antidiabetic medications alone. These patients should be identified and provided with insulin in a timely manner. An additional reason that patients may have to transition from oral agents to insulin is the occurrence of specific side effects or contraindications for certain patients. For example, weight gain and edema are general side effects associated with the use of thiazolidinediones and this class of oral agents should not be used in patients at high risk for heart failure.19,20 Use of metformin in patients with significant renal dysfunction may be associated with lactic acidosis, and metformin is contraindicated in patients with congestive heart failure, significant liver disease, or any condition predisposing hypoperfusion.21,22 Side effects associated with α-glucosidase inhibitors include intestinal gas, abdominal cramps, and diarrhea, although, symptoms usually subside with time.23,24 Therefore, contraindications and/or side effects associated with various oral antidiabetic agents may be an additional factor that necessitates transition to insulin therapy.
In addition to insulin as add-on therapy, a new class of agents known as the incretin mimetics is being studied in type 2 diabetes patients. While not yet approved for use by FDA, exenatide is the first drug in this class and is initially being evaluated to improve glycemic control in patients who are not using insulin and are not achieving glycemic goals with oral agents.25 A series of phase 3 trials, collectively known as the AC2993: Diabetes Management for Improving Glucose Outcomes (AMIGO) studies, demonstrated that the addition of exenatide to existing metformin and/or sulfonylurea regimens allowed more patients to achieve glycemic goals relative to patients randomized to placebo.26-28 While additional and larger studies that compare exenatide to other oral agents and to insulin are needed, these results demonstrate that exenatide is effective as an adjuvant therapy in patients who do not achieve glycemic control on metformin and/or a sulfonylurea.
A new and unique injectable agent, pramlintide (Symlin, Amylin), was recently approved by FDA for use prior to meals in insulin-treated patients with type 2 and type 1 diabetes. This synthetic analog of the naturally occurring pancreatic hormone amylin helps to reduce postprandial rises in glucose by 2 mechanisms. First, it delays gastric emptying, thereby slowing down the absorption of nutrients following a meal. This may also enhance satiety. Second, it suppresses secretion of glucagon, the pancreatic hormone that opposes the effects of insulin and acts to raise blood glucose levels.
IDENTIFYING PATIENTS WITH CHANGING NEEDS It appears that the longer the duration of oral antidiabetic therapy in patients with type 2 diabetes, the more likely the regimen will fail to achieve glycemic control.29 In a study conducted by the UKPDS Group, only 50% of patients with type 2 diabetes who received treatment with sulfonylurea monotherapy were able to maintain A1C levels <7.0% after 3 years of therapy.29 As the length of treatment increased, the proportion of patients able to achieve this level of glycemic control steadily decreased from 34% after 6 years of therapy to 24% after 9 years of therapy.29 Because treatment failure can occur over a relatively short period of time, it is important to remain vigilant and regularly monitor patients to evaluate changes in maintenance of glycemic control on current therapies. Of those patients failing more rapidly with oral agents, some are actually found to have an indolent form of autoimmune or type 1 diabetes, commonly referred to as latent autoimmune diabetes of adulthood. Anti-γ amino decarboxylase antibodies were found in about 10% of patients in the UKPDS with newly diagnosed type 2 diabetes.
Glycemic control is best evaluated by using a combination of results from A1C testing and patient self-monitoring of blood glucose.1 ADA recommends regular monitoring of A1C measurements at 3-month intervals in patients with type 2 diabetes to determine if values fall within the recommended range for metabolic control.1 In order to achieve target A1C levels, it also is important to monitor fasting plasma glucose (FPG) through patient self-monitoring of blood glucose.1 FPG levels have been strongly correlated with A1C measurements, as have postprandial glucose (PPG) levels.30,31 The American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) recommend targeting FPG levels of <110 mg/dL and 2-hour PPG levels of <140 mg/dL while ADA has recommended PPG levels of 90 to 130 mg/dL and PPG levels of <180 mg/dL in patients with diabetes.1,14 Reviewing A1C measurements and self-monitored blood glucose values with patients at office visits provides physicians with an opportunity to identify when adjustments to diabetes management plans are needed. To this end, patients should be encouraged to self-monitor blood glucose levels. Patients whose blood glucose levels are changing rapidly may need to test 2 to 3 times/d and record results in blood glucose diaries, online meter upload and tracking devices, or electronic log sheets. In contrast, very stable patients may not require testing every day. However, varying testing times, including postprandial, remains important for adequate assessment. Examination of these patterns often is very important in deciding the next step in treatment.
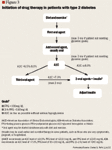
Therapy should always be intensified when it fails to maintain glycemic control to an A1C <7.0%1 if following ADA recommendations, and an A1C ≤6.5% if following AACE recommendations. When adequate glycemic control is not achieved in patients who already are receiving optimum dosages of current oral antidiabetic medications, either another oral antidiabetic agent should be added to the therapeutic regimen or insulin should be initiated (Figure 2).32 No more than 3 months should be allowed to elapse without modifying the therapeutic regimen of patients who are not meeting glycemic targets. The amount of time required to determine the effectiveness of a given therapeutic regimen tends to vary according to the particular agent that was added most recently. Sulfonylureas can typically be titrated to a maximum dosage in 2 to 3 weeks, whereas approximately 4 weeks are required for metformin and as many as 3 months are required for the thiazolidinediones.18,33
INITIATING INSULIN THERAPY For patients who continue to have A1C levels >7.0% on 2 oral antidiabetic agents after a maximum of 3 months of treatment, intensification of therapy is required. Those patients who have A1C levels of <8.0% may benefit from either the addition of a third oral antidiabetic agent, such as thiazolidinedione, or insulin (Figure 2). For patients in whom therapy with 3 oral antidiabetic mediations is still inadequate in lowering A1C levels to <7.0%, insulin should be added. Insulin therapy should almost always be initiated in patients whose A1C levels remain above 8% to 9% despite taking 2 oral antidiabetic agents, since the addition of a thiazolidinedione to the therapeutic regimen is unlikely to reduce A1C more than 1.0% to 2.0%.14,18,34,35

Additionally, cost savings may be achieved by adding insulin instead of a second or third oral agent to existing oral therapy. For example, thiazolidinediones are utilized widely as a second or third oral agent,18 and combination therapy with a sulfonylurea and a thiazolidinedione can be fairly expensive.37 Notably, other oral therapy combinations, such as metformin plus a thiazolidinedione, a sulfonylurea plus acarbose, or meglitinide plus metformin, can be even more expensive than combination therapy with glimepiride and a thiazolidinedione,38 and the addition of a third oral agent would only further compound costs. However, insulin therapy, even in combination with an oral agent, can be relatively inexpensive compared with regimens that include combinations of oral agents. For instance, a 100-U/mL, 10-mL vial of insulin glargine is $58.42 and a daily dose of 47 units39 would cost $2.75/d (along with the nominal cost of syringes).37 Combination therapy with glimepiride and insulin glargine would cost about $3.92 to $5.08/d.37 Thus, adding insulin therapy to an oral regimen that alone is no longer effective in maintaining glycemic control can be considerably less expensive than sequential addition of oral therapies to maximum doses (in the examples given above, up to 55% less).
There are several strategies for initiating insulin therapy in patients with type 2 diabetes, each having advantages and limitations. Split mixed or premixed regimens are mixtures of regular insulin and long-acting insulin usually administered twice daily. Mixed insulin formulations attempt to cover insulin peaks at mealtimes and are sometimes preferred because they are relatively convenient and simple to use. However, these insulin regimens do not allow for much flexibility, and meals must be taken on a more regular schedule. Hypoglycemia may occur if a meal is missed, an issue that may be particularly challenging with premixed formulations since insulin components cannot be adjusted separately. In a recent study by Raskin et al, insulin glargine at bedtime was compared with twice-daily 70% longer-acting protamine aspart and 30% insulin aspart mix, plus metformin and continuation of a thiazolidinedione, if applicable. Insulin secretagogues were discontinued in all treatment groups. Notably, patients in the glargine treatment group were not receiving any prandial insulin. The authors of this study point out that discontinuation of secretagogues may have disadvantaged the insulin glargine treatment arm. Final A1C values were better with the pre-mix in patients whose starting A1C exceeded 8.5% (10.2%-7.1% vs 10.1%-7.7%, P<.05), but were the same when the initial A1C was <8.5%. Regardless, usage of the pre-mix in both of these groups caused more hypoglycemia, more weight gain, and a substantially larger insulin dose.40 This study demonstrates that once-daily insulin glargine is as effective if not superior to twice-daily pre-mixed insulins in achieving glycemic control in patients with values to 8.5% and is associated with less hypoglycemia. It is also difficult to ascertain what the comparison would be with an A1C >8.5%, since the discontinuation of the insulin secretagogue is counter to the usual recommended practice.
Patients who require insulin may be started alternatively on basal insulin therapy. Available intermediate and long-acting basal insulins include Lente (Humulin L, Lilly), neutral protamine Hagedorn (NPH) (Humulin N, Lilly), Ultralente (Humulin U, Lilly), and insulin glargine (Lantus, Aventis). Lente and NPH insulins are generally provided in a twice-daily regimen. While Ultralente insulin has been used as a basal insulin, it has a relatively unpredictable time-action profile and currently is not used widely in the United States.41 Insulin glargine is a newer long-acting insulin that provides a relatively constant action profile over a 24-hour period.41 It may be given once daily and has the advantage of flexible dosing, appearing to be effective when given either at bedtime or in the morning.42 Bedtime basal insulin provided in combination with oral antidiabetic agents is generally a convenient strategy for starting insulin. This basal insulin plus oral therapy combination appears to improve outcomes and prolong the duration of glucose control compared with bedtime basal insulin alone.43,44

An ideal insulin regimen would mimic normal pancreatic secretion of insulin, providing coverage throughout the day, including mealtimes. Glycemic control may be optimized by replacement of both basal and prandial components of insulin. While there is evidence that basal- prandial regimens provide superior glycemic control compared with either basal or prandial insulin alone,16 these regimens may be difficult to implement for patients initially starting insulin.
A relatively simple approach to starting insulin has been recently evaluated in which bedtime basal insulin is introduced in a forced titration schedule to patients with type 2 diabetes who are continuing therapy with oral antidiabetic medications.39 In a study of 756 overweight patients with type 2 diabetes (A1C levels >7.5%) who were taking≤2 oral antidiabetics, the effects of bedtime insulin glargine were compared with NPH when added to ongoing oral antihyperglycemic therapy.39 A forced (continued until the target is achieved) weekly insulin dose titration schedule was employed in which insulin doses were increased by 2 to 8 IU/d based on mean self-monitored FPG values, targeting FPG levels of ≤100 mg/dL.39 The majority of patients in this study were successful in achieving recommended targets for glycemic control after only 24 weeks of therapy, with mean A1C levels <7.0% in both treatment groups.39 However, significantly more patients treated with insulin glargine achieved this goal without an episode of documented nocturnal hypoglycemia compared with those who received NPH (33.2% vs 26.7%, P<.05).39 Additionally, the insulin glargine group reported significantly fewer episodes of symptomatic hypoglycemia throughout the study period, compared with patients who received NPH insulin (13.9 vs 17.7 events/patient year, P<.02).39 This simplified approach to initiating insulin therapy appears to be effective in improving glycemic control, while the reduced incidence of nocturnal hypoglycemia seen with insulin glargine may also help address this potential barrier to starting insulin in patients with diabetes.39
BARRIERS TO INSULIN THERAPY Despite overwhelming evidence supporting the benefits of insulin in patients with type 2 diabetes, many physicians and patients continue to have negative perceptions about insulin and remain reluctant to start therapy even when it is indicated.46 Physicians are often concerned about the side effects associated with insulin, particularly hypoglycemia and weight gain, as well as the practical difficulties of incorporating insulin training for patients into their clinical practice. Many patients share these concerns about the side effects of insulin and their ability to correctly administer injections.45 Also, patients are often concerned about the inconveniences and discomfort perceived to be associated with insulin injections.45 Some patients feel that being prescribed insulin means that their disease has advanced into a very serious stage, whereas not being given insulin indicates that they are still in relatively good health.45 The need for insulin may also signify to some patients a personal failure to adequately care for themselves.45 These relatively prevalent barriers to insulin therapy may cause a delay in starting treatment when it is needed, exposing patients to a temporal gap in glycemic control.

CONCLUSIONS The health-care burden associated with diabetes has become an increasing threat to both the patient and society as a result of high morbidity, mortality, and economic costs associated with this disease. It is of paramount importance that glycemic control be achieved and maintained in patients with newly diagnosed disease to avoid the serious complications that may occur as a result of chronic hyperglycemia. While oral antidiabetic therapy may be effective initially for providing some degree of glycemic control in patients with newly diagnosed diabetes, such treatment often does not achieve target levels of glucose control and generally is unable to maintain glycemic control indefinitely. Over time, insulin therapy eventually becomes necessary to maintain glycemic control in the vast majority of patients.
Dr Dailey is clinical professor at the University of California, San Diego School of Medicine, San Diego, Calif, and head of diabetes research and senior consultant, Division of Diabetes & Endocrinology, Scripps Clinic, La Jolla, Calif. He can be reached at gdailey@scrippsclinic.com
Disclosures: The author reports the following activities: investigator for Bristol-Myers Squibb, GlaxoSmithKline, sanofi-aventis, Eli Lilly, Schering-Plough, Merck, and Amylin; consultant for sanofi-aventis, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Novo Nordisk, and Amylin; speaker for sanofi-aventis, Bristol-Myers Squibb, GlaxoSmithKline, Eli Lilly, Wyeth, and Amylin. The preparation of this manuscript was supported in part by Aventis Pharmaceuticals, a member of the sanofi-aventis Group.
REFERENCES 1. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28:S4-S6.
2. American Diabetes Association. National Diabetes Fact Sheet: General information and national estimates on diabetes in the United States, 2002. Available at: http:// http://www.diabetes.org/. Accessed March 14, 2005.
3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
4. Polonsky KS. Lilly Lecture 1994. The beta-cell in diabetes: from molecular genetics to clinical research. Diabetes. 1995;44:705-717.
5. Hogan P, Dall T, Nikolov P, and the American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
6. The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517-523.
7. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563-2569.
8. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
9. Nathan DM, Lachin J, Cleary P, et al, and the Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294-2303.
10. Cleary P, Orchard T, Zinman B, and the DCCT/EDIC study group. Coronary calcification in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort [abstract]. Presented at: The 63rd Annual Scientific Sessions of the American Diabetes Association; June 13-17, 2003; New Orleans, La. Abstract 652-P.
11. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
12. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24: 1815-1820.
13. Roy R, Navar M, Palomeno G, Davidson MB. Real world effectiveness of rosiglitazone added to maximal (tolerated) doses of metformin and a sulfonylurea agent: a systematic evaluation of triple oral therapy in a minority population. Diabetes Care. 2004;27:1741-1742.
14. American Association of Clinical Endocrinologists and the American College of Endocrinology. The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: The AACE System of Intensive Diabetes Self-Management-2002 update. Endocr Pract. 2002;8(suppl 1):40-82
15. Buse JB. Overview of current therapeutic options in type 2 diabetes. Rationale for combining oral agents with insulin therapy. Diabetes Care. 1999;22 Suppl 3:C65-C70.
16. Bergenstal RM, Kendall DM, Franz MJ, Rubenstein AH. Management of Type 2 Diabetes: a Systematic Approach to Meeting the Standards of Care. II: Oral Agents, Insulin, and Management of Complications. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 4th ed. Philadelphia, Pa: W.B. Saunders Co: 2001:821-835.
17. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR, and the UK Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25: 330-336.
18. Bell DS, Ovalle F. Long-term efficacy of triple oral therapy for type 2 diabetes mellitus. Endocr Pract. 2002;8:271-275.
19. Avandia [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
20. Actos [package insert]. Lincolnshire, IL: Takeda Pharmaceuticals America; 2004.
21. Glucophage/Glucophage XR [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2004.
22. Glucotrol XL [prescribing information]. New York, NY: Pfizer, Inc.; 2003.
23. Precose [prescribing information]. West Haven, CT: Bayer Pharmaceuticals Corporation; 2004.
24. Glyset [prescribing information]. New York, NY: Pharmacia & Upjohn Company; 2004.
25. Gryskiewicz KA, Coleman CI. Focus on exenatide: A novel incretin mimetic hormone for the treatment of type 2 diabetes. Formulary. 2005;40:86-90.
26. Defronzo R, Ratner R, Han J, Kim D, Fineman M, Baron AD. Effects of exenatide (synthetic exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes [abstract]. Presented at: The 64th Scientific Sessions of the American Diabetes Association; June 4-8, 2004; Orlando, Fla. Abstract 6.
27. Buse JB, Henry RR, Han J, et al, and the Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
28. Kendall DM, Riddle MC, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight in patients with type 2 diabetes treated with metformin and a sulfonylurea [abstract]. Presented at: The 64th Annual Scientific Sessions of the American Diabetes Association; June 4-8, 2004; Orlando, Fla. Abstract 10.
29. Turner RC, Cull CA, Frighi V, Holman RR, and the UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999; 281:2005-2012.
30. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881-885.
31. Rohlfing CL, Wiedmeyer HM, Little RR, England JG, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275-278.
32. Optimizing insulin therapy in type 2 diabetes. Proceedings from: Optimizing Control: Redefining Intensive Management of Type 2 Diabetes Mellitus roundtable; July 12-13, 2003; New York, NY.
33. Garber AJ, Donovan DS Jr, Dandona P, Bruce S, Park JS. Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab. 2003;88: 3598-3604.
34. Dailey GE 3rd, Noor MA, Park JS, Bruce S, Fiedorek FT. Glycemic control with glyburide/metformin tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized, double-blind trial. Am J Med. 2004;116:223-229.
35. Yale JF, Valiquett TR, Ghazzi MN, Owens-Grillo JK, Whitcomb RW, Foyt HL. The effect of a thiazolidinedione drug, troglitazone, on glycemia in patients with type 2 diabetes mellitus poorly controlled with sulfonylurea and metformin. A multicenter, randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134: 737-745.
36. Rosenblum MS, Kane MP. Analysis of cost and utilization of health care services before and after initiation of insulin therapy in patients with type 2 diabetes mellitus. J Manag Care Pharm. 2003;9:309-316.
37. 2004 Red Book: Pharmacy's Fundamental Reference. Montvale, NJ: Thomson Medical Economics; 2004.
38. Kabadi UM. Cost-effective management of hyperglycemia in patients with type 2 diabetes using oral agents. Managed Care. 2004;13:48-49, 53-56, 58-59.
39. Riddle MC, Rosenstock J, Gerich J, and the Insuline Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26: 3080-3086.
40. Raskin P, Allen E, Hollander P, et al, and the INITIATE Study Group. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005; 28:260-265.
41. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
42. Fritsche A, Schweitzer MA, Häring HU, and the 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138: 952-959.
43. Fritsche A, Schm� RM, Häring HU, Stumvoll M. Intensive insulin therapy combined with metformin in obese type 2 diabetic patients. Acta Diabetol. 2000;37:13-18.
44. Ponssen HH, Elte JWF, Lehert P, Schouten JP, Bets D. Combined metformin and insulin therapy for patients with type 2 diabetes mellitus. Clin Ther. 2000;22:709-718.
45. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients' fears and hopes about insulin therapy. The basis of patient reluctance. Diabetes Care. 1997;20:292-298.
46. Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653-1659.
47. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
48. Meneghini L, Wick A, Delamater A, et al. Group-based self-management intervention for intensification of insulin therapy [abstract]. Presented at: The 63rd Annual Scientific Sessions of the American Diabetes Association; June 13-17, 2003; New Orleans, La. Abstract 132-OR.
49. Murphy NP, Keane SM, Ong KK, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care. 2003;26:799-804.
50. Raskin P, Klaff L, Bergenstal R, Halle JP, Donley D, Mecca T. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care. 2000;23:1666-1671.
51. Novolog [package insert]. Princeton, NJ: Novo Nordisk Pharmaceuticals Inc; 2004.
52. Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care. 2000;23:583-588.
53. Apidra [package insert]. Kansas City, MO: sanofi-aventis; 2004.
54. Taylor R, Davies R, Fox C, Sampson M, Weaver JU, Wood L. Appropriate insulin regimes for type 2 diabetes: a multicenter randomized crossover study.
55. Lantus [package insert]. Kansas City, MO: sanofi-aventis; 2003.
