- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Too early to predict impact of biosimilars on utilization of hematopoietic growth factors
Overall, it would not appear that the new erythropoiesis-stimulating agents for treatment of cytopenia will add substantially to pharmacy budgets. As they are approved by FDA, each medication will need to be reviewed to understand its complete clinical advantage, and the safety profile will need to be completely understood.

Key Points
Hematopoietic growth factors are used to treat a variety of inherited or acquired cytopenias. These cytopenias can be caused by congenital diseases, idiopathic diseases, malignancies, chronic kidney disease, and myelosuppression related to chemotherapy. Thus, hematopoietic growth factors are prescribed by multiple clinicians representing many disciplines.
Currently approved products (darbepoetin alfa, romiplostim, eltrombopag, and plerixafor) are being studied for new indications. Other agents in development include an erythropoeisis-stimulating agent, Hematide; a thrombopoiesis-stimulating agent, AKR-501; the granulopoiesis-stimulating agents Neutroval and ezatiostat (Telintra); and a lymphopoiesis-stimulating agent, CYT104.
There is skepticism that the products in development, both new entities and potential new indications for products already on the market, will offer any real advantages over the currently available products. It is premature to predict the impact of biosimilars on utilization of hematopoietic growth factors; the biosimilars may not be interchangeable with currently available products and side effects may be a concern. However, we do know that because of cost and usage across multiple disciplines, appropriate use will continue to be paramount in managing cytopenias.
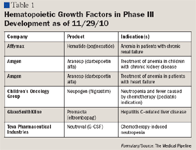
Several existing therapies are being tested for extended indications. Some of these agents seem to offer some end-point improvements, but more analysis of the studies to determine the significance of these improvements is needed.
The new erythropoiesis-stimulating agents would replace existing therapy; therefore, the cost impact should not be substantial. The thrombopoiesis-stimulating agents have some interesting potential, particularly if they can assist patients who have human immunodeficiency virus infection from losing antiretroviral activity. These new agents would be an additional therapy to existing regimens, so their costs would be additive in nature.
Overall, it would not appear that the new therapies will add substantially to pharmacy budgets. As they are approved by FDA, each medication will need to be reviewed to understand its complete clinical advantage, and the safety profile will need to be completely understood. New agents likely will be covered and managed on fourth tier, along with the existing medications. Prior authorization is likely to ensure that the desired use is consistent with an FDA-approved indication. Whether step therapy is used will depend on the cost of the new agent compared with that of existing drugs and the complete clinical profile.
–Steven G. Avey, MS, RPh, vice president, national sales, Regence Rx, Portland, OR
Copyright 2011 by The Medical Pipeline, LLC. All rights reserved. For more information about The Medical Pipeline, please visit http://www.tmp-pipeline.com/.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
