- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Travoprost: A prostaglandin analogue for the treatment of glaucoma
In August 2003, the US Department of Veterans Affairs (VA) awarded a contract for prostaglandin ophthalmic agents with travoprost as the agent of choice. Although there was no national mandate to switch patients from existing therapy, many VA facilities had agreements from their local ophthalmology and optometry departments to conduct a therapeutic interchange of patients from existing prostaglandins (eg, latanoprost) to travoprost.

Key Points
Abstract
In August 2003, the US Department of Veterans Affairs (VA) awarded a contract for prostaglandin ophthalmic agents with travoprost as the agent of choice. Although there was no national mandate to switch patients from existing therapy, many VA facilities had agreements from their local ophthalmology and optometry departments to conduct a therapeutic interchange of patients from existing prostaglandins (eg, latanoprost) to travoprost. The primary purpose of this report is to describe the experience of conversion to travoprost after this contract. Medical records of converted patients were evaluated for change in intraocular pressure (IOP), change in visual field, adverse events, and whether or not a change back to the original therapy or alternative therapy was required. Of 578 patients evaluated, most (84%) had been using latanoprost before the conversion. After conversion, mean IOP was significantly reduced by 1.64 ± 4.95 mm Hg (P<.0001). Of 188 patients with documented visual field assessment, 89.9% had no change or improvement in visual field after conversion. Adverse events occurred in 44 (7.6%) patients, with hyperemia occurring in 13 (2.2%) patients. Discontinuation of travoprost was attributed to an adverse event in 29 (5.0%) patients and attributed to ineffectiveness in 17 (2.9%) patients. Adverse drug event rates were significantly less than those reported in the literature. Travoprost was effective and well tolerated in most patients after therapeutic interchange. This evaluation provides additional information regarding outcomes associated with the therapeutic interchange. (Formulary. 2009; 44:322-328.)
Glaucoma affects 15 million people, resulting in 12,000 new cases of blindness per year in the United States and is the second leading cause of blindness worldwide.1,5 Many factors influence the development of glaucoma. It is more prevalent in people aged more than 40 years and is 6 to 8 times more common in blacks than whites.1,2 Additionally, family history of glaucoma, elevated intraocular pressure (IOP), systemic vascular disease, and diabetes are risk factors for glaucoma development.3
Reduction of IOP may be accomplished with drugs, laser therapy, or surgery. Topically applied ocular agents are usually the first step in the management of glaucoma.
Currently, there are 5 classes of medications that are used to lower eye pressure: topical cholinergic agonists, topical beta blockers, topical adrenergic agonists, topical prostaglandin analogues, and topical and oral inhibitors of carbonic anhydrase.4 Prostaglandin analogues are the newest class of topical glaucoma medications approved by FDA and have proven to be safe and effective for lowering IOP. The 4 topical prostaglandin analogues available in the United States are latanoprost, bimatoprost, travoprost, and unoprostone. The most common adverse event experienced with these agents is hyperemia, although the incidence may differ among agents.5 In a head-to-head comparison of latanoprost, travoprost, and bimatoprost, there were few patient withdrawals from the trial because of adverse events.5
Latanoprost was the first ophthalmic prostaglandin approved by FDA in 1996. The introduction of 3 other ophthalmic prostaglandin analogues (bimatoprost, travoprost, and unoprostone) in 2000 and 2001 prompted the Veterans Affairs Pharmacy Benefits Management (VA PBM) group to perform a class review of the ophthalmic prostaglandins. The review determined that unoprostone was less effective than the other agents and that latanoprost, bimatoprost, and travoprost were similar in efficacy and tolerability.6 Latanoprost had been the ophthalmic prostaglandin preferred within the VA system at that time. The VA PBM concluded that glaucoma could be appropriately treated with any 1 of the 3 similar agents and recommended contracting with 1 agent to be the formulary ophthalmic prostaglandin of choice
In August 2003, this contract was awarded to travoprost. The contract required that all new prescriptions for an ophthalmic prostaglandin be travoprost rather than another agent in the class. The other agents were placed on nonformulary status and required acquisition of a nonformulary use approval before dispensing. VA facilities were not required to perform a therapeutic interchange from other ophthalmic prostaglandins to travoprost, although many facilities chose to do so. This retrospective observational review was conducted to assess the tolerability and adverse effects of travoprost after conversion from another ophthalmic prostaglandin.
OBJECTIVES
The primary purpose of this study was to describe the experience of a VA multisite therapeutic interchange to travoprost as the prostaglandin ophthalmic agent of choice after it had been awarded a VA national contract. The study was designed to evaluate IOP after conversion to travoprost; to evaluate for change in the visual field, as documented in the medical progress notes, after conversion to travoprost; to collect information on methods of conversion implementation used by VA sites; to monitor for any adverse effects after conversion to travoprost therapy (visual disturbances, hyperemia, tearing discomfort, etc.); to collect information on patient tolerability of adverse effects and willingness to continue therapy; and to define the number of changes back to original or alternate prostaglandin therapy after travoprost therapy and reasons for such action.
METHODS
This was a retrospective observational cohort analysis, with data collected by 14 investigators at 6 VA facilities. Permission to perform the study was obtained from the investigational review board at each facility. Potential participants for the study were identified via analysis of the VA electronic prescription database, which contains extensive information on all prescriptions obtained through the VA system. Potential participants must have been a recipient of a new prescription for travoprost between August 1, 2003, and April 1, 2005, and a recipient of at least one 30-day prescription of latanoprost or bimatoprost before conversion to travoprost. After identification, participants were randomly selected from each participating VA facility. A projected sample size of 500 patients was based on the expected event rates (50% for adverse events and 5% for switch rates) and the precision desired in the estimates (95% confidence intervals of 46%-54% and 3%-7%, respectively). Patients were excluded from analysis if there were evidence or a history of ocular surgery or laser intervention or other ocular pathology except glaucoma and ocular hypertension.
Each patient's medical record was reviewed from the start of travoprost therapy through at least 6 months of follow-up, with data extraction to include age; sex; race; glaucoma subtype; travoprost start date; method of conversion to travoprost; concurrent ophthalmic medications; concurrent ophthalmic diagnoses; presence of seasonal allergies; discontinuation of travoprost and reason for discontinuation; agent initiated after travoprost discontinuation, if applicable; IOP measurements in the 6 months before and after travoprost initiation; serial visual field readings after travoprost initiation; adverse drug events (ADEs); and unscheduled visits because of ADEs.
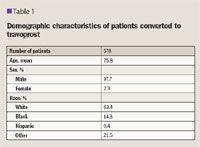
RESULTS
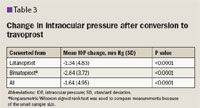
The IOP in black patients after conversion decreased by –2.81 mm Hg compared with patients who were not black (–1.48 mm Hg) but was not statistically significant.
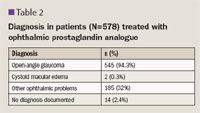
Visual field assessment was documented in 188 (32.5%) patient records after the conversion to travoprost.
Of those with documented visual field assessment, 108 (57.4%) reported no change in visual field, 61 (32.4%) reported improvement in visual field, and 19 (10.1%) reported worsening of visual field.
Of the patients converted to travoprost, 147 (25.4%) discontinued travoprost treatment, and 35 (6.1%) patients were treated with a different prostaglandin analogue.
Thirty-seven (6.4%) patients died during the study period and were included in the reasons for discontinuation of travoprost data.
Of the patients that discontinued travoprost, an ADE was the reason for 29 (20%) patients, and ineffectiveness was the reason for 17 (12%) patients.
Hyperemia was noted to be the reason for discontinuation in 10 patients and eye pain for 12 patients.
Other ADEs included headache, blepharitis, dizziness, dry eyes, follicular reaction, hypersensitivity, inflammation, trichiasis, and any event that was documented as unknown.
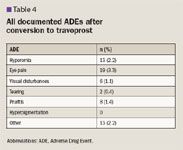
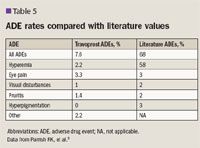
The total number of patients converted to travoprost who experienced hyperemia was significantly less than those reported in published data.5 Rates of eye pain, visual disturbances, hyperpigmentation, and other categories were similar to the published literature with no significant differences.
The study population had significantly lower combined ADE rates compared to published literature.
At the time the VA contract was awarded to travoprost, the daily costs of ophthalmic prostaglandin analogues per patient were $0.522 for bimatoprost and $0.634 for latanoprost compared to $0.145 for travoprost.
Additional cost savings analysis estimates this voluntary conversion to travoprost saved the VA $10.8 million annually in drug costs.
DISCUSSION
This retrospective review presents the results of a therapeutic interchange to travoprost in selected VA patients.
Overall efficacy was maintained in most patients, relatively few ADEs were reported, and drug cost savings to the VA was more than $10 million annually.
Intraocular pressure was significantly lower after conversion to travoprost among those patients with paired measurements and was significant whether converting from latanoprost or bimatoprost.
Additionally, most patients with documented visual field assessment showed no change. Published studies of ophthalmic prostaglandin analogues have found similar efficacy when comparing latanoprost, bimatoprost, and travoprost.
Although 1 trial reported travoprost to be superior to latanoprost in lowering IOP in black patients, our study did not denote a significant difference in IOP reduction.5,7,8
For most patients, the change to travoprost was well tolerated.
Although more than a quarter of the patients discontinued travoprost during the study period, the reasons were infrequently related to safety.
We found the overall ADE rate to be less than that reported in a previously published trial.5 The study design and provider documentation could account for this difference.
Our study had several strengths. An additional benefit of conducting an observational study of this nature was in developing a model to study other therapeutic interchange programs.
Our study sites were geographically diverse with patients from both coasts and north, south, and central United States.
Despite lack of complete documentation in approximately half of our patients, we still maintained a relatively large sample size, with longer follow-up time for measuring effectiveness of the medication.
Although results of this study of therapeutic interchange appear reassuring, it should be noted there are several limitations inherent with retrospective, observational trials.
Most significant was inconsistent documentation in the medical record.
Both baseline and follow-up IOP measurements were documented in only slightly more than half of the medical records, and visual field assessment during the follow-up period was documented in only one-third of the medical records. Inconsistent documentation might be a contributor to the low incidence of ADEs reported in our study.
Other study limitations include lack of assessment of patient adherence to therapy and possible variance in interpretation of information found in the medical record, because multiple investigators were involved in data collection.
We sought to minimize the variable interpretation during data collection through monthly investigator conference calls, a frequently asked question sheet, and an email group to provide a forum for investigator discussions.
The low rates of ADEs or lack of efficacy and the overall decrease in IOP in evaluable patients suggest that the conversion program was successful.
Although therapeutic conversions are a common practice among pharmacy benefits management programs, relatively few organizations report outcomes associated with the process.
Hence, our report adds to the literature and serves as a model to study other therapeutic interchange programs in terms of safety and efficacy.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
Traci C. Brooks, PharmD, Womack Army Medical Center, Fort Bragg, NC; North Florida/South Georgia Veterans Health System, Gainesville, FL; Mark Burlingame, PharmD, BCPS, program director of primary care, North Florida/South Georgia Veterans Health System, Gainesville, FL; Muriel Burk, PharmD, VACO PBM Services/VA Center for Medication Safety, Hines, IL; Kathy Tortorice, PharmD, BCPS, VACO Pharmacy Benefits Management Services, Hines, IL; Diane Dong, BSN, MS, VACO PBM Services/VA Center for Medication Safety, Hines, IL; Peter A. Glassman, MBBS, MSc, VA Greater Los Angeles Healthcare System/David Geffen School of Medicine at UCLA, Los Angeles, CA; Jennie A. Lynch, PharmD, VISN 2 VA Healthcare Network Upstate New York, Syracuse, NY; Chester B. Good, MD, MPH, VA Pittsburgh Healthcare System/University of Pittsburg Schools of Medicine and Pharmacy, Pittsburgh, PA; Holly S. Rickman, MS, PharmD, Central Arkansas Veterans Healthcare System, Little Rock, AR; Melanie Claborn, PharmD, University of Arkansas for Medical Sciences/Veterans Healthcare System of the Ozarks Fayetteville, AR; Duva Appleman, MD, Northern California Veterans Healthcare System/University of California, Davis Mather, CA; William Jones, MS, FASHP, VACO PBM Services and Southwest Consolidated Mail Outpatient Pharmacy, Tucson, AZ; Lanita Shaverd, PharmD, Central Arkansas Veterans Healthcare System, Little Rock, AR
Acknowledgments: Tom Emmendorfer, PharmD, VACO PBM Services/VA Center for Medication Safety, Hines, IL.
REFERENCES
1. Quigley HA, Vitale S. Models of Open-Angle Glaucoma Prevalence and Incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83-91.
2. Glaucoma Research Foundation. http://www.glaucoma.org/learn/glaucoma_facts.php/. Accessed October 15, 2009.
3. Georgopoulos G, Andreanos D. Liokis N, et al. Risk factors in ocular hypertension. Eur J Ophthamol. 1997;7:357-363.
4. Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411-434. Erratum in: Drugs. 2002;62:1314.
5. Parrish RK, Palmberg P, Sheu WP; XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135:688-703.
6. VHA Pharmacy Benefits Management Strategic Healthcare Group and the Medial Advisory Panel. Drug Class Review: Ophthalmic Prostaglandin Analogs. June 2002. http://www.pbm.va.gov/reviews/ophthprostopthreview5.pdf. Accessed September 24, 2009.
7. Netland PA, Landry T, Sullivan EK, et al.; Travoprost Study Group. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132:472-484.
8. Kumar RS, Istiantoro VW, Hoh ST, et al. Efficacy and safety of a systematic switch from latanoprost to travoprost in patients with glaucoma. J Glaucoma. 2007;16:606-609.
New Study Planned for Bevacizumab to Treat AMD
December 20th 2023Outlook Therapeutics plans to begin a study in the first quarter of 2024 to address the issues identified in a FDA complete response letter. If approved, Lytenava would be the only bevacizumab product to specifically treat age-related macular degeneration.
