- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Unfavorable risk:benefit ratio with antiplatelet plus anticoagulant therapy for peripheral arterial disease
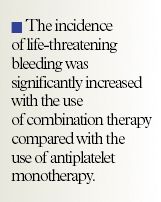
In a randomized, controlled, open-label trial in patients with peripheral arterial disease (PAD), it was demonstrated that antiplatelet therapy plus an oral anticoagulant was no better at preventing major cardiovascular complications than antiplatelet therapy alone. The combination therapy was also associated with a significant increase in life-threatening bleeding complications compared with monotherapy.

Key Points
These results from The Warfarin Antiplatelet Vascular Evaluation (WAVE) trial were published in the New England Journal of Medicine (NEJM).
A total of 2,161 patients aged 35 to 85 years with PAD were randomized to receive either antiplatelet monotherapy consisting of aspirin, ticlopidine, or clopidogrel (n=1,081) or antiplatelet therapy plus warfarin or acenocoumarol (n=1,080). The target international normalized ratio (INR) was 2.0 to 3.0. The coprimary end points of the trial included 1) the combined end point of myocardial infarction (MI), stroke, or death from cardiovascular causes and 2) the combined end point of MI, stroke, severe ischemia of peripheral or coronary arteries requiring immediate intervention, or death from cardiovascular causes. Patients were also assessed for their risk of bleeding (classified as life-threatening, moderate, or minor, depending on clinical presentation).
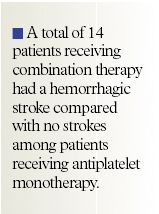
The authors stated that these bleeding risks are notable, as the study excluded all patients at higher risk for bleeding, such as those taking long-term nonsteroidal anti-inflammatory drugs (NSAIDs) and those who had a previous history of gastrointestinal bleeding or recent stroke. In addition, all potential participants in the study were required to complete a 2 to 4 week run-in period before randomization to ensure their ability to maintain a stable INR within therapeutic range during the clinical trial.
The authors stated that "On the basis of the WAVE results, oral anticoagulation combined with anti-platelet therapy is not indicated in patients with peripheral arterial disease, since no significant benefit was observed and substantial risk was incurred." They continued by stressing that "Treating 1,000 patients with combination therapy as compared with antiplatelet therapy alone for 3 years would lead to 24 fewer cardiovascular events but 28 more episodes of life-threatening bleeding, resulting in a net increase in serious adverse outcomes."
In an accompanying editorial, Emile R. Mohler III, MD, stated that studies with analogous outcomes related to increased bleeding with combination antiplatelet and anticoagulant therapy have been published in the past. Dr Mohler agreed with the conclusions reached by the WAVE trial researchers regarding the relative safety of mono-therapy over combination therapy and stated that "Further information on the pathobiologic basis for bleeding in patients with peripheral arterial disease is needed to develop successful clinical strategies to prevent bleeding and to devise safer antiplatelet and anticoagulant drugs."
SOURCES
Anand S, Yusuf S, Xie C, et al; for The Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357:217–227.
Mohler ER III. Atherothrombosis-Wave goodbye to combined anticoagulation and antiplatelet therapy [editorial]? N Engl J Med. 2007;357:293–296.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
