- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Ustekinumab: A human monoclonal antibody for the treatment of plaque psoriasis
Ustekinumab is a novel investigational human monoclonal antibody (mAb) that is pending approval for the treatment of plaque psoriasis. Subcutaneous administration of ustekinumab has demonstrated efficacy in both phase 2 and 3 trials.
Abstract
Ustekinumab is a novel investigational human monoclonal antibody (mAb) that is pending approval for the treatment of plaque psoriasis. As a chronic inflammatory skin disorder, psoriasis can be effectively treated with biologic agents, which target immune pathways. Current approved biologic agents for psoriasis include agents that inhibit tumor necrosis factor (TNF)-alpha (eg, etanercept, infliximab, adalimumab) or T-cell activation (eg, alefacept, efalizumab). Ustekinumab works through the inhibition of interleukin (IL)-12 and IL-23. Subcutaneous administration of ustekinumab has demonstrated efficacy in both phase 2 and 3 trials, with larger proportions of ustekinumab-treated patients (>50%) achieving 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with placebo-treated patients. Ustekinumab-treated patients also experienced greater improvements in health-related quality of life. Ustekinumab is generally well tolerated; the most commonly reported adverse events include cough, arthralgia, injection-site reaction, and headache. (Formulary. 2009;44:72–76.)
Psoriasis is a chronic inflammatory skin disorder that affects approximately 2% to 3% of the world's population. The most common form of the disease is plaque psoriasis, which is a result of dysregulated cell growth. Plaque psoriasis presents as symmetrical, sharply demarcated erythematous regions covered with silvery-white scales on the scalp, trunk, and limbs.1,2 The presence of psoriatic plaques is often triggered or exacerbated by environmental conditions including infection, physical or psychological stress, cold weather, and medications.1 Because of patients' concerns regarding their appearance and the physical discomfort associated with plaques, patients often experience deceases in their health-related quality-of-life (HRQOL).1
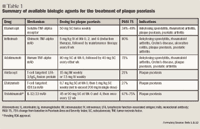
The newest biologic agent in development, ustekinumab (Centocor), is the first fully human monoclonal antibody (mAb) directed at interleukin (IL)-12 and IL-23; this agent binds these cytokines and blocks their inflammatory activity.3 Centocor filed a BLA for ustekinumab for the treatment of moderate-to-severe chronic plaque psoriasis in 2007. In June 2008, FDA's Dermatologic and Ophthalmic Drugs Advisory Committee unanimously recommended approval of ustekinumab. In December 2008, FDA issued a complete response letter for this agent, asking for additional information on the proposed Risk Evaluation and Mitigation Strategy (REMS).
CHEMISTRY & PHARMACOLOGY
The formation of psoriatic plaques involves the interplay of T cells, cytokines, and keratinocytes (the major cell type of the epidermis layer of the skin). In an immune response, IL-12 and IL-23 are involved in the activation of T cells. These cytokines are presented to T cells by dendritic cells or macrophages and bind to T-cell surface receptors IL12R-beta-2 and IL23R. Psoriatic lesions have an increased expression of both IL-12 and IL-23, which suggests the involvement of these cytokines in the development of psoriatic plaques.4–6 IL-12 activates a population of helper T cells, which are responsible for the secretion of interferon-gamma and TNF-alpha, cytokines that are key players in psoriasis pathophysiology.7 IL-23 activates another T-cell subtype that begins a cascade of cytokine release, which causes hyperproliferation of keratinocytes.8
Ustekinumab is a human IgG mAb that binds to the p40 subunit, which is common to both IL-12 and IL-23. The binding of ustekinumab to IL-12 and IL-23 blocks the cytokine interaction with T-cell surface receptors and subsequently blocks the immune cascade that would result from such interaction.
PHARMACOKINETICS

CLINICAL TRIALS
Evaluation of therapy. The Psoriasis Area and Severity Index (PASI) provides a uniform method to describe the severity of disease based on body surface area involvement, erythema, induration, and scaling and is commonly used in clinical trials of psoriasis treatments to assess efficacy.1 The score is based on the assessment of extent of involvement in 4 major anatomical regions (head, trunk, arms, and legs), as well as the severity of desquamation, erythema, and plaque thickness in each region.10 The PASI score ranges from 0 (no disease) to 72 (maximal disease). An improvement of 75% from baseline (PASI 75) is commonly used as the dichotomous cut-off for clinical response in most trials.1 In addition to the PASI 75 end point, the Physicians Global Assessment (PGA) has also been used to assess psoriasis drug efficacy in clinical trials. The PGA is a scale by which the physician grades the severity of disease after treatment; the proportion of patients with "clear" or "excellent" skin ("minimal disease") is typically assessed.1 The scale ranges from 1 (clear) to 7 (severe). The Dermatology Life Quality Index (DLQI), a 10-item questionnaire, is also used in clinical trials.1 Scores range from 0 to 30, with 0 representing no disease impact on HRQOL (a favorable score).1 Clinically significant changes in DLQI scores, also called the minimally important differences (MID), are estimated to range between 2.3 and 5.7.11
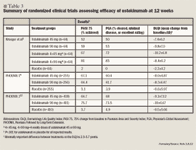
Phase 3 trials. The phase 3 trials investigating ustekinumab have been dubbed the Psoriasis Followed by Long-Term Extension (PHOENIX) 1 and PHOENIX 2 trials, both of which were randomized, double-blind, placebo-controlled trials in patients with moderate-to-severe plaque psoriasis (defined as a baseline PASI score of ≥12).8,12
In the PHOENIX 1 trial, patients (N=766) were randomly assigned to receive ustekinumab 45 or 90 mg at Weeks 0 and 4 and every 12 weeks thereafter or placebo at Weeks 0 and 4, with 50% of patients randomized to cross over to ustekinumab 45 mg and 50% to ustekinumab 90 mg at Week 12.8 At Week 40, patients who had initially been randomized to receive ustekinumab and who achieved the PASI 75 end point at Weeks 28 and 40 were re-randomized to either continue maintenance treatment with ustekinumab or to be withdrawn from active treatment (placebo). Patients withdrawn from active treatment at Week 40 were re-treated when they lost ≥50% of PASI improvement. Patients not achieving PASI 75 at Week 28 or 40 were not re-randomized, and their dosing was discontinued or modified.
At Week 12, greater proportions of ustekinumab-treated patients (at both doses) achieved the PASI 75 end point or were rated clear or minimal (excellent) on the PGA compared with placebo-treated patients (P<.0001 for all comparisons).8 Likewise, DLQI scores were both statistically and clinically significantly decreased (improved) in the ustekinumab groups compared with the placebo group at 12 weeks.8 The effects of ustekinumab were sustained with maintenance therapy over ≥1 year of continued treatment, as defined by the time to loss of PASI 75 response, but response declined after discontinuation of ustekinumab in the placebo group.8
The methodology of the first 28 weeks of the PHOENIX 1 and 2 trials were exactly the same, but PHOENIX 2 (N=1,230) studied the intensification of dose frequency in patients who only partially responded to the initial 12 weeks of therapy.12 At Week 28, partial responders (defined as patients who had initially been randomized to ustekinumab treatment and achieved the PASI 50 end point but not PASI 75) were re-randomized to either continue receiving the study drug every 12 weeks or to receive intensified dosing every 8 weeks.12

ADVERSE EVENTS
The most commonly reported adverse events associated with ustekinumab treatment in clinical trials were cough, arthralgia, injection-site reaction, and headache.3,8,12 Studies have demonstrated that there are no dose-related increases in adverse events with ustekinumab treatment. Approximately 5% of patients develop antibodies to ustekinumab, but the relationship between antibody development and adverse events is unclear.3,8,12 The development of anti-ustekinumab antibodies could potentially lead to an immune reaction, such as serum sickness, or to a decreased response to therapy.
Because of the immunosuppressant effects of ustekinumab, patients are at increased risk of infection during therapy. In the phase 2 trial, 2 ustekinumab-treated patients developed severe infections (cellulitis and pneumonia).3 Furthermore, 2 cases of severe infection occurred in the ustekinumab group of the PHOENIX 1 trial (cellulitis and herpes zoster), but both resolved with appropriate therapy.8
In the PHOENIX 2 trial, patients who received the more intensified ustekinumab dosing schedule (every 8 wk) experienced common adverse events, such as cough, arthralgia, headache, and injection-site reaction, at a higher rate than those who received ustekinumab every 12 weeks (73% vs 63%; P=not reported). However, there was no increase in the incidence of serious adverse events (3% vs 7%; P=not reported).12
DRUG INTERACTIONS
Limited published data are available regarding drug interactions with ustekinumab. This agent has only been investigated as monotherapy in patients with moderate-to-severe psoriasis, and concomitant medications for other indications were not reported in clinical trials.3,8,12 Further investigations must be performed to assess the potential for drug interactions with ustekinumab.
DOSING AND ADMINISTRATION
FDA has not yet approved ustekinumab; however, phase 3 trials have demonstrated clinical efficacy (defined as achievement of the PASI 75 end point) with SC ustekinumab 45 and 90 mg administered at Weeks 0 and 4 and then every 12 weeks thereafter.8,12 In the PHOENIX 2 trial, SC ustekinumab 90 mg as a maintenance dose every 8 weeks was beneficial in initial partial responders.12 The manufacturer has proposed weight-based dosing of ustekinumab; patients <100 kg should receive ustekinumab 45 mg, and patients ≥100 kg should receive ustekinumab 90 mg.
Dr Phung is an outcomes research fellow, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr Coleman is assistant professor of Pharmacy Practice, University of Connecticut School of Pharmacy, and methods chief and program director, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr White is professor of Pharmacy Practice, University of Connecticut School of Pharmacy, and director, University of Connecticut/Hartford Hospital Evidence-Based Practice Center.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008; 58:826–850.
2. Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004; 113:1664–1675.
3. Krueger GG, Langley RG, Leonardi C, et al; CNTO 1275 Psoriasis Study Group. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592.
4. Yawalkar N, Karlen S, Hunger R, Brand CU, Braathen LR. Expression of interleukin-12 is increased in psoriatic skin. J Invest Dermatol. 1998; 111:1053–1057.
5. Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130.
6. Chen X, Tan Z, Yue Q, Liu H, Liu Z, Li J. The expression of interleukin-23 (p19/p40) and interleukin-12 (p35/p40) in psoriasis skin. J Huazhong Univ Sci Technolog Med Sci. 2006;26:750–752.
7. Cytokines. In: Goldsby RA, Kindt TJ, Osborne BA, Kuby J, eds. Immunology. 5th ed. New York, NY: W.H. Freeman and Company; 2003:276–298.
8. Leonardi CL, Kimball AB, Papp KA, et al; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) [erratum in Lancet. 2008;371:1838]. Lancet. 2008;371:1665–1674.
9. Gottlieb AB, Cooper KD, McCormick TS, et al. A phase 1, double-blind, placebo-controlled study evaluating single subcutaneous administrations of a human interleukin-12/23 monoclonal antibody in subjects with plaque psoriasis. Curr Med Res Opin. 2007;23:1081–1092.
10. Fredriksson T, Pettersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica. 1978;157:238–244.
11. Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: Results of a phase II study. Health Qual Life Outcomes. 2006;4:71–83.
12. Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675–1684.
Scripius Removes Dupixent from Formulary
September 7th 2023The decision by Scripius (previously Select Health Prescriptions) was made based on the cost of Dupixent, as well as because of an increased demand to use Dupixent for mild atopic dermatitis when the primary patient population is those with moderate to severe disease.
