- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Vildagliptin and rosiglitazone demonstrate similar efficacy, tolerability in patients with type 2 diabetes
Results of a double-blind, randomized, contolled trial of vildagliptin versus rosiglitazone demonstrate similar efficacy and tolerability in patients with type 2 diabetes.

Key Points
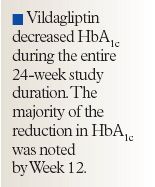
The primary efficacy end point in this study was the change from baseline in HbA1c at study end using the last observation carried forward for those patients who discontinued early. Secondary efficacy end points included changes in fasting plasma glucose (FPG) level, fasting plasma lipid value, and body weight. Noninferiority of vildagliptin versus rosiglitazone on HbA1c effect was established if the upper limit of the 95% CI for between-treatment difference was not >0.4%.
Patients were randomized to vildagliptin or rosiglitazone in a 2:1 ratio. Of the 786 patients randomized, 697 were included in the intent-to-treat (ITT) population (vildagliptin, n=459; rosiglitazone, n=238). Approximately one-third of patients in the ITT population had a baseline HbA1c>9%, and approximately 30% had a body mass index ≥35 kg/m2 . Patients were predominantly Caucasian, with a mean age of 54 years and a mean disease duration of 2.4 years.

During the study, a similar percentage of patients in both treatment groups reported $1 adverse event. In vildagliptin-treated patients, the most common adverse events were nasopharyngitis (6.8%), dizziness (6%), headache (5%), and upper respiratory tract infection (4.5%). In rosiglitazone-treated patients, the most frequent adverse events were nasopharyngitis (7.5%), headache (5.2%), dizziness (4.1%), and peripheral edema (4.1%). In vildagliptin-treated patients, the incidence of peripheral edema was 2.1%. One patient in each treatment group reported 1 mild hypoglycemic reaction. Study discontinuations due to adverse events were similar between the 2 treatment groups (vildagliptin, 2.9%; rosiglitazone, 3.4%).
These study results demonstrated that vildagliptin was well tolerated and caused no weight gain, with HbA1c-lowering effects similar to those observed with rosiglitazone. Compared with rosiglitazone, treatment with vildagliptin had more favorable lipid effects, with significant improvements in LDL, non-HDL, and total cholesterol; triglycerides; and total-to-HDL cholesterol ratio. These 2 agents were similarly well tolerated (with the exception of edema and weight gain with rosiglitazone), and both demonstrated a low incidence of hypoglycemia.
SOURCE Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes. Diabetes Care. 2007;30:217–223.
